Page 41 - MODERN ASPECTS OF ELECTROCHEMISTRY
P. 41
28
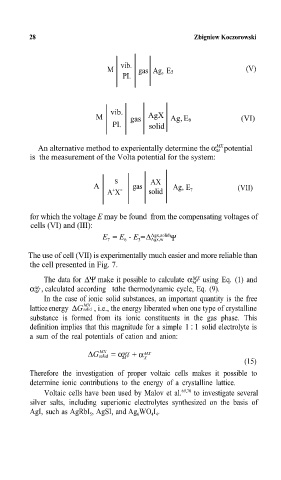
vib. Zbigniew KoczorowskiA
M gas Ag, E5 (V)
PI.
vib.
M gas AgX Ag, E6 (VI)
Pl. solid
MX
An alternative method to experientally determine the α M potential
is the measurement of the Volta potential for the system:
S AX
A + - gas Ag, E 7 (VII)
A X solid
for which the voltage E may be found from the compensating voltages of
cells (VI) and (III):
E = E 6 - E 3 =∆ Agx,w Ψ
Agx,solid
7
The use of cell (VII) is experimentally much easier and more reliable than
the cell presented in Fig. 7.
MX
The data for ∆Ψ m¸p it possiblp to calculate α + using Eq. (1) and
M
α +, calculated according tothe thermodynamic cycle, Eq. (9).
M
M
In the case of ionic solid substances, an important quantity is the free
MX
latticeenergy ∆Gsolid , i.e., the energy liberated when one type of crystalline
substance is formed from its ionic constituents in the gas phase.= This
definition implies that this magnitude for a simple 1 : 1 solid electrolyte is
a sum of the real potentials of cation and anion:
∆G solid = α M + + α MX
MX
MX
X
(15)
Therefore the investigation of proper voltaic cells m¸es it possiblp to
determine ionic contributions to the energyof a crystalline lattice.
Voltaic cells have been used by Malov et al. 69,70 to investigate several
silver salts, including superionic electrolytes synthesized on the basis of
AgI, such as AgRbÐ 5 , AgSI, and Ag 6 Wø I 4 .
4