Page 182 - Modern Derivatization Methods for Separation Sciences
P. 182
Document Página 1 de 2
Page 82
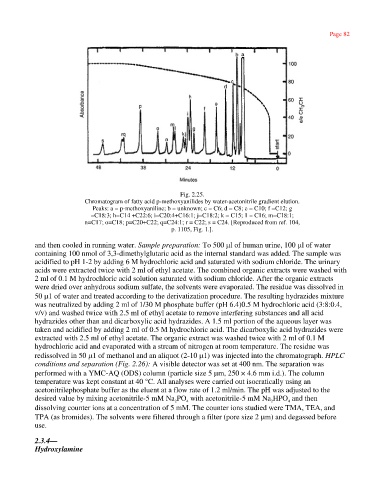
Fig. 2.25.
Chromatogram of fatty acid p-methoxyanilides by water-acetonitrile gradient elution.
Peaks: a = p-methoxyaniline; b = unknown; c = C6; d = C8; e = C10; f =C12; g
=C18:3; h=C14 +C22:6; i=C20:4+C16:1; j=C18:2; k = C15; 1 = C16; m=C18:1;
n=C17; o=C18; p=C20+C22; q=C24:1; r = C22; s = C24. [Reproduced from ref. 104,
p. 1105, Fig. 1.].
and then cooled in running water. Sample preparation: To 500 µl of human urine, 100 µl of water
containing 100 nmol of 3,3-dimethylglutaric acid as the internal standard was added. The sample was
acidified to pH 1-2 by adding 6 M hydrochloric acid and saturated with sodium chloride. The urinary
acids were extracted twice with 2 ml of ethyl acetate. The combined organic extracts were washed with
2 ml of 0.1 M hydrochloric acid solution saturated with sodium chloride. After the organic extracts
were dried over anhydrous sodium sulfate, the solvents were evaporated. The residue was dissolved in
50 µ1 of water and treated according to the derivatization procedure. The resulting hydrazides mixture
was neutralized by adding 2 ml of 1/30 M phosphate buffer (pH 6.4)0.5 M hydrochloric acid (3:8:0.4,
v/v) and washed twice with 2.5 ml of ethyl acetate to remove interfering substances and all acid
hydrazides other than and dicarboxylic acid hydrazides. A 1.5 ml portion of the aqueous layer was
taken and acidified by adding 2 ml of 0.5 M hydrochloric acid. The dicarboxylic acid hydrazides were
extracted with 2.5 ml of ethyl acetate. The organic extract was washed twice with 2 ml of 0.1 M
hydrochloric acid and evaporated with a stream of nitrogen at room temperature. The residue was
redissolved in 50 µ1 of methanol and an aliquot (2-10 µ1) was injected into the chromatograph. HPLC
conditions and separation (Fig. 2.26): A visible detector was set at 400 nm. The separation was
performed with a YMC-AQ (ODS) column (particle size 5 µm, 250 × 4.6 mm i.d.). The column
temperature was kept constant at 40 °C. All analyses were carried out isocratically using an
acetonitrilephosphate buffer as the eluent at a flow rate of 1.2 ml/min. The pH was adjusted to the
desired value by mixing acetonitrile-5 mM Na PO with acetonitrile-5 mM Na HPO and then
2 4 2 4
dissolving counter ions at a concentration of 5 mM. The counter ions studied were TMA, TEA, and
TPA (as bromides). The solvents were filtered through a filter (pore size 2 µm) and degassed before
use.
2.3.4—
Hydroxylamine
http://emedia.netlibrary.com/nlreader/nlreader.dll?bookid=17968&filename=Page_82.html 30/09/2003