Page 184 - Modern Derivatization Methods for Separation Sciences
P. 184
Document Página 1 de 2
Page 83
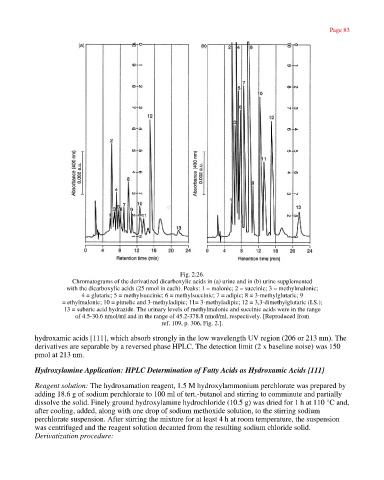
Fig. 2.26.
Chromatograms of the derivatized dicarboxylic acids in (a) urine and in (b) urine supplemented
with the dicarboxylic acids (25 nmol in each). Peaks: 1 = malonic; 2 = succinic; 3 = methylmalonic;
4 = glutaric; 5 = methylsuccinic; 6 = methylsuccinic; 7 = adipic; 8 = 3-methylglutaric; 9
= ethylmalonic; 10 = pimelic and 3-methyladipic; 11= 3-methyladipic; 12 = 3,3-dimethylglutaric (I.S.);
13 = suberic acid hydrazide. The urinary levels of methylmalonic and succinic acids were in the range
of 4.5-30.6 nmol/ml and in the range of 45.2-378.8 nmol/ml, respectively. [Reproduced from
ref. 109, p. 306, Fig. 2.].
hydroxamic acids [111], which absorb strongly in the low wavelength UV region (206 or 213 nm). The
derivatives are separable by a reversed phase HPLC. The detection limit (2 x baseline noise) was 150
pmol at 213 nm.
Hydroxylamine Application: HPLC Determination of Fatty Acids as Hydroxamic Acids [111]
Reagent solution: The hydroxamation reagent, 1.5 M hydroxylammonium perchlorate was prepared by
adding 18.6 g of sodium perchlorate to 100 ml of tert.-butanol and stirring to comminute and partially
dissolve the solid. Finely ground hydroxylamine hydrochloride (10.5 g) was dried for 1 h at 110 °C and,
after cooling, added, along with one drop of sodium methoxide solution, to the stirring sodium
perchlorate suspension. After stirring the mixture for at least 4 h at room temperature, the suspension
was centrifuged and the reagent solution decanted from the resulting sodium chloride solid.
Derivatization procedure:
http://emedia.netlibrary.com/nlreader/nlreader.dll?bookid=17968&filename=Page_83.html 30/09/2003