Page 186 - Modern Derivatization Methods for Separation Sciences
P. 186
Document Página 1 de 2
Page 84
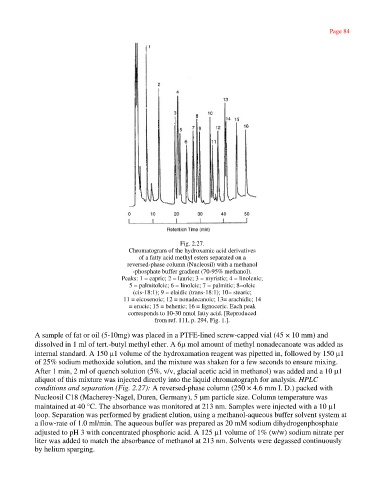
Fig. 2.27.
Chromatogram of the hydroxamic acid derivatives
of a fatty acid methyl esters separated on a
reversed-phase column (Nucleosil) with a methanol
-phosphate buffer gradient (70-95% methanol).
Peaks: 1 = capric; 2 = lauric; 3 = myristic; 4 = linolenic;
5 = palmitoleic; 6 = linoleic; 7 = palmitic; 8=oleic
(cis-18:1); 9 = elaidic (trans-18:1); 10= stearic;
11 = eicosenoic; 12 = nonadecanoic; 13= arachidic; 14
= erucic; 15 = behenic; 16 = lignoceric. Each peak
corresponds to 10-30 nmol fatty acid. [Reproduced
from ref. 111, p. 294, Fig. 1.].
A sample of fat or oil (5-10mg) was placed in a PTFE-lined screw-capped vial (45 × 10 mm) and
dissolved in 1 ml of tert.-butyl methyl ether. A 6µ mol amount of methyl nonadecanoate was added as
internal standard. A 150 µ1 volume of the hydroxamation reagent was pipetted in, followed by 150 µ1
of 25% sodium methoxide solution, and the mixture was shaken for a few seconds to ensure mixing.
After 1 min, 2 ml of quench solution (5%, v/v, glacial acetic acid in methanol) was added and a 10 µ1
aliquot of this mixture was injected directly into the liquid chromatograph for analysis. HPLC
conditions and separation (Fig. 2.27): A reversed-phase column (250 × 4.6 mm I. D.) packed with
Nucleosil C18 (Macherey-Nagel, Duren, Germany), 5 µm particle size. Column temperature was
maintained at 40 °C. The absorbance was monitored at 213 nm. Samples were injected with a 10 µ1
loop. Separation was performed by gradient elution, using a methanol-aqueous buffer solvent system at
a flow-rate of 1.0 ml/min. The aqueous buffer was prepared as 20 mM sodium dihydrogenphosphate
adjusted to pH 3 with concentrated phosphoric acid. A 125 µ1 volume of 1% (w/w) sodium nitrate per
liter was added to match the absorbance of methanol at 213 nm. Solvents were degassed continuously
by helium sparging.
http://emedia.netlibrary.com/nlreader/nlreader.dll?bookid=17968&filename=Page_84.html 30/09/2003