Page 134 - MODERN ELECTROCHEMISTRY
P. 134
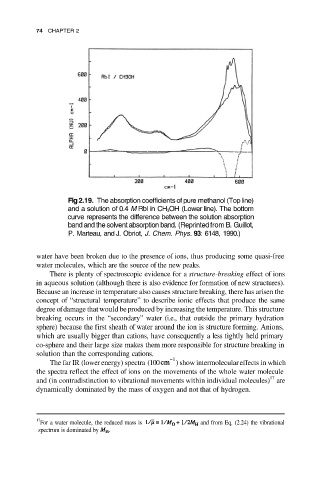
74 CHAPTER 2
Fig 2.19. The absorption coefficients of pure methanol (Top line)
and a solution of 0.4 M Rbl in CH 3OH (Lower line). The bottom
curve represents the difference between the solution absorption
band and the solvent absorption band. (Reprinted from B. Guillot,
P. Marteau, and J. Obriot, J. Chem. Phys. 93: 6148, 1990.)
water have been broken due to the presence of ions, thus producing some quasi-free
water molecules, which are the source of the new peaks.
There is plenty of spectroscopic evidence for a structure-breaking effect of ions
in aqueous solution (although there is also evidence for formation of new structures).
Because an increase in temperature also causes structure breaking, there has arisen the
concept of “structural temperature” to describe ionic effects that produce the same
degree of damage that would be produced by increasing the temperature. This structure
breaking occurs in the “secondary” water (i.e., that outside the primary hydration
sphere) because the first sheath of water around the ion is structure forming. Anions,
which are usually bigger than cations, have consequently a less tightly held primary
co-sphere and their large size makes them more responsible for structure breaking in
solution than the corresponding cations.
The far IR (lower energy) spectra (100 ) show intermolecular effects in which
the spectra reflect the effect of ions on the movements of the whole water molecule
17
and (in contradistinction to vibrational movements within individual molecules) are
dynamically dominated by the mass of oxygen and not that of hydrogen.
17
For a water molecule, the reduced mass is and from Eq. (2.24) the vibrational
spectrum is dominated by