Page 137 - MODERN ELECTROCHEMISTRY
P. 137
ION–SOLVENT INTERACTIONS 77
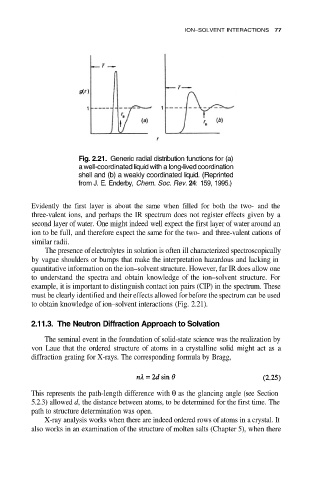
Fig. 2.21. Generic radial distribution functions for (a)
a well-coordinated liquid with a long-lived coordination
shell and (b) a weakly coordinated liquid. (Reprinted
from J. E. Enderby, Chem. Soc. Rev. 24: 159, 1995.)
Evidently the first layer is about the same when filled for both the two- and the
three-valent ions, and perhaps the IR spectrum does not register effects given by a
second layer of water. One might indeed well expect the first layer of water around an
ion to be full, and therefore expect the same for the two- and three-valent cations of
similar radii.
The presence of electrolytes in solution is often ill characterized spectroscopically
by vague shoulders or bumps that make the interpretation hazardous and lacking in
quantitative information on the ion–solvent structure. However, far IR does allow one
to understand the spectra and obtain knowledge of the ion–solvent structure. For
example, it is important to distinguish contact ion pairs (CIP) in the spectrum. These
must be clearly identified and their effects allowed for before the spectrum can be used
to obtain knowledge of ion–solvent interactions (Fig. 2.21).
2.11.3. The Neutron Diffraction Approach to Solvation
The seminal event in the foundation of solid-state science was the realization by
von Laue that the ordered structure of atoms in a crystalline solid might act as a
diffraction grating for X-rays. The corresponding formula by Bragg,
This represents the path-length difference with θ as the glancing angle (see Section
5.2.3) allowed d, the distance between atoms, to be determined for the first time. The
path to structure determination was open.
X-ray analysis works when there are indeed ordered rows of atoms in a crystal. It
also works in an examination of the structure of molten salts (Chapter 5), when there