Page 141 - MODERN ELECTROCHEMISTRY
P. 141
ION–SOLVENT INTERACTIONS 81
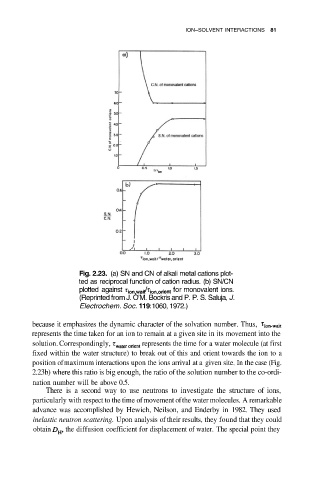
Fig. 2.23. (a) SN and CN of alkali metal cations plot-
ted as reciprocal function of cation radius. (b) SN/CN
plotted against for monovalent ions.
(Reprinted from J. O’M. Bockris and P. P. S. Saluja, J.
Electrochem. Soc. 119:1060, 1972.)
because it emphasizes the dynamic character of the solvation number. Thus,
represents the time taken for an ion to remain at a given site in its movement into the
solution. Correspondingly, represents the time for a water molecule (at first
fixed within the water structure) to break out of this and orient towards the ion to a
position of maximum interactions upon the ions arrival at a given site. In the case (Fig.
2.23b) where this ratio is big enough, the ratio of the solution number to the co-ordi-
nation number will be above 0.5.
There is a second way to use neutrons to investigate the structure of ions,
particularly with respect to the time of movement of the water molecules. A remarkable
advance was accomplished by Hewich, Neilson, and Enderby in 1982. They used
inelastic neutron scattering. Upon analysis of their results, they found that they could
obtain the diffusion coefficient for displacement of water. The special point they