Page 142 - MODERN ELECTROCHEMISTRY
P. 142
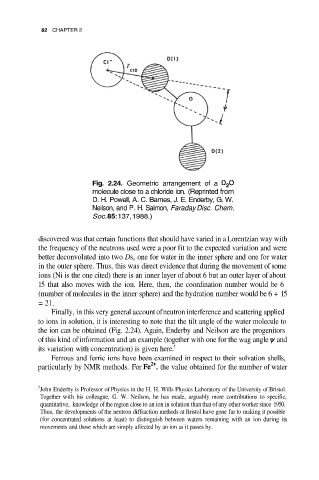
82 CHAPTER 2
Fig. 2.24. Geometric arrangement of a
molecule close to a chloride ion. (Reprinted from
D. H. Powell, A. C. Barnes, J. E. Enderby, G. W.
Neilson, and P. H. Salmon, Faraday Disc. Chem.
Soc. 85:137, 1988.)
discovered was that certain functions that should have varied in a Lorentzian way with
the frequency of the neutrons used were a poor fit to the expected variation and were
better deconvoluted into two Ds, one for water in the inner sphere and one for water
in the outer sphere. Thus, this was direct evidence that during the movement of some
ions (Ni is the one cited) there is an inner layer of about 6 but an outer layer of about
15 that also moves with the ion. Here, then, the coordination number would be 6
(number of molecules in the inner sphere) and the hydration number would be 6 + 15
= 21.
Finally, in this very general account of neutron interference and scattering applied
to ions in solution, it is interesting to note that the tilt angle of the water molecule to
the ion can be obtained (Fig. 2.24). Again, Enderby and Neilson are the progenitors
of this kind of information and an example (together with one for the wag angle and
its variation with concentration) is given here. †
Ferrous and ferric ions have been examined in respect to their solvation shells,
particularly by NMR methods. For the value obtained for the number of water
†
John Enderby is Professor of Physics in the H. H. Wills Physics Laboratory of the University of Bristol.
Together with his colleague, G. W. Neilson, he has made, arguably more contributions to specific,
quantitative, knowledge of the region close to an ion in solution than that of any other worker since 1950.
Thus, the developments of the neutron diffraction methods at Bristol have gone far to making it possible
(for concentrated solutions at least) to distinguish between waters remaining with an ion during its
movements and those which are simply affected by an ion as it passes by.