Page 139 - MODERN ELECTROCHEMISTRY
P. 139
ION–SOLVENT INTERACTIONS 79
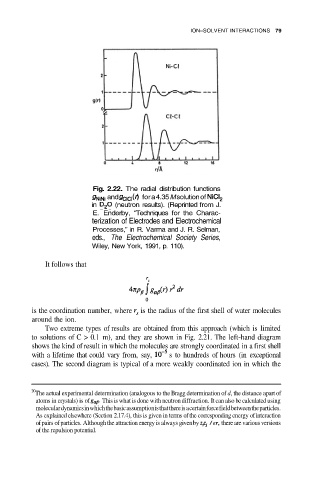
Fig. 2.22. The radial distribution functions
and for a 4.35 M solution of
in (neutron results). (Reprinted from J.
E. Enderby, “Techniques for the Charac-
terization of Electrodes and Electrochemical
Processes,” in R. Varma and J. R. Selman,
eds., The Electrochemical Society Series,
Wiley, New York, 1991, p. 110).
It follows that
is the coordination number, where is the radius of the first shell of water molecules
around the ion.
Two extreme types of results are obtained from this approach (which is limited
to solutions of C > 0.1 m), and they are shown in Fig. 2.21. The left-hand diagram
shows the kind of result in which the molecules are strongly coordinated in a first shell
with a lifetime that could vary from, say, s to hundreds of hours (in exceptional
cases). The second diagram is typical of a more weakly coordinated ion in which the
20
The actual experimental determination (analogous to the Bragg determination of d, the distance apart of
atoms in crystals) is of This is what is done with neutron diffraction. It can also be calculated using
molecular dynamics in which the basic assumption is that there is a certain force field between the particles.
As explained elsewhere (Section 2.17.4), this is given in terms of the corresponding energy of interaction
of pairs of particles. Although the attraction energy is always given by there are various versions
of the repulsion potential.