Page 203 - MODERN ELECTROCHEMISTRY
P. 203
140 CHAPTER 2
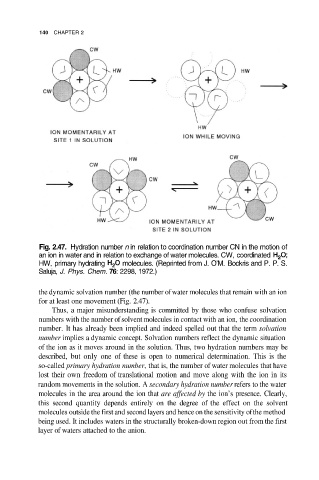
Fig. 2.47. Hydration number n in relation to coordination number CN in the motion of
an ion in water and in relation to exchange of water molecules. CW, coordinated
HW, primary hydrating molecules. (Reprinted from J. O’M. Bockris and P. P. S.
Saluja, J. Phys. Chem. 76: 2298, 1972.)
the dynamic solvation number (the number of water molecules that remain with an ion
for at least one movement (Fig. 2.47).
Thus, a major misunderstanding is committed by those who confuse solvation
numbers with the number of solvent molecules in contact with an ion, the coordination
number. It has already been implied and indeed spelled out that the term solvation
number implies a dynamic concept. Solvation numbers reflect the dynamic situation
of the ion as it moves around in the solution. Thus, two hydration numbers may be
described, but only one of these is open to numerical determination. This is the
so-called primary hydration number, that is, the number of water molecules that have
lost their own freedom of translational motion and move along with the ion in its
random movements in the solution. A secondary hydration number refers to the water
molecules in the area around the ion that are affected by the ion’s presence. Clearly,
this second quantity depends entirely on the degree of the effect on the solvent
molecules outside the first and second layers and hence on the sensitivity of the method
being used. It includes waters in the structurally broken-down region out from the first
layer of waters attached to the anion.