Page 201 - MODERN ELECTROCHEMISTRY
P. 201
138 CHAPTER 2
2.15.14. Is There a Connection between the Entropy of Solvation and
the Heats of Hydration?
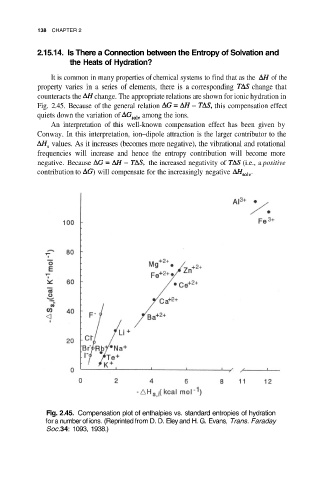
It is common in many properties of chemical systems to find that as the of the
property varies in a series of elements, there is a corresponding change that
counteracts the change. The appropriate relations are shown for ionic hydration in
Fig. 2.45. Because of the general relation this compensation effect
quiets down the variation of among the ions.
An interpretation of this well-known compensation effect has been given by
Conway. In this interpretation, ion–dipole attraction is the larger contributor to the
values. As it increases (becomes more negative), the vibrational and rotational
frequencies will increase and hence the entropy contribution will become more
negative. Because the increased negativity of (i.e., a positive
contribution to ) will compensate for the increasingly negative
Fig. 2.45. Compensation plot of enthalpies vs. standard entropies of hydration
for a number of ions. (Reprinted from D. D. Eley and H. G. Evans, Trans. Faraday
Soc.34: 1093, 1938.)