Page 205 - MODERN ELECTROCHEMISTRY
P. 205
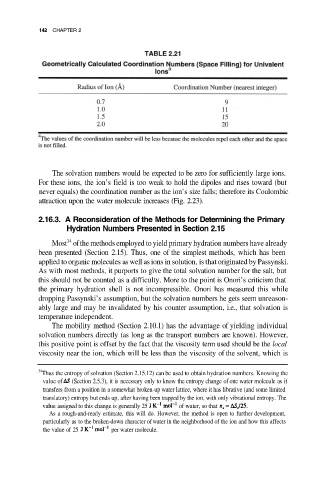
142 CHAPTER 2
The solvation numbers would be expected to be zero for sufficiently large ions.
For these ions, the ion’s field is too weak to hold the dipoles and rises toward (but
never equals) the coordination number as the ion’s size falls; therefore its Coulombic
attraction upon the water molecule increases (Fig. 2.23).
2.16.3. A Reconsideration of the Methods for Determining the Primary
Hydration Numbers Presented in Section 2.15
34
Most of the methods employed to yield primary hydration numbers have already
been presented (Section 2.15). Thus, one of the simplest methods, which has been
applied to organic molecules as well as ions in solution, is that originated by Passynski.
As with most methods, it purports to give the total solvation number for the salt, but
this should not be counted as a difficulty. More to the point is Onori’s criticism that
the primary hydration shell is not incompressible. Onori has measured this while
dropping Passynski’s assumption, but the solvation numbers he gets seem unreason-
ably large and may be invalidated by his counter assumption, i.e., that solvation is
temperature independent.
The mobility method (Section 2.10.1) has the advantage of yielding individual
solvation numbers directly (as long as the transport numbers are known). However,
this positive point is offset by the fact that the viscosity term used should be the local
viscosity near the ion, which will be less than the viscosity of the solvent, which is
34
Thus the entropy of solvation (Section 2.15.12) can be used to obtain hydration numbers. Knowing the
value of (Section 2.5.3), it is necessary only to know the entropy change of one water molecule as it
transfers from a position in a somewhat broken-up water lattice, where it has librative (and some limited
translatory) entropy but ends up, after having been trapped by the ion, with only vibrational entropy. The
value assigned to this change is generally 25 of water, so that
As a rough-and-ready estimate, this will do. However, the method is open to further development,
particularly as to the broken-down character of water in the neighborhood of the ion and how this affects
the value of 25 per water molecule.