Page 210 - MODERN ELECTROCHEMISTRY
P. 210
146 CHAPTER 2
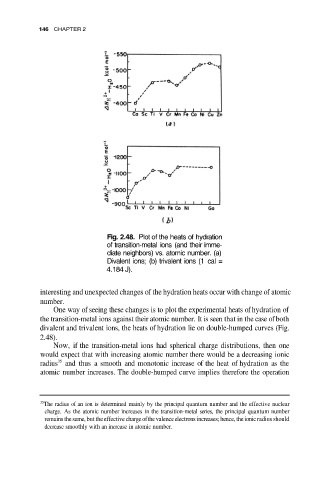
Fig. 2.48. Plot of the heats of hydration
of transition-metal ions (and their imme-
diate neighbors) vs. atomic number. (a)
Divalent ions; (b) trivalent ions (1 cal =
4.184 J).
interesting and unexpected changes of the hydration heats occur with change of atomic
number.
One way of seeing these changes is to plot the experimental heats of hydration of
the transition-metal ions against their atomic number. It is seen that in the case of both
divalent and trivalent ions, the heats of hydration lie on double-humped curves (Fig.
2.48).
Now, if the transition-metal ions had spherical charge distributions, then one
would expect that with increasing atomic number there would be a decreasing ionic
35
radius and thus a smooth and monotonic increase of the heat of hydration as the
atomic number increases. The double-humped curve implies therefore the operation
35 The radius of an ion is determined mainly by the principal quantum number and the effective nuclear
charge. As the atomic number increases in the transition-metal series, the principal quantum number
remains the same, but the effective charge of the valence electrons increases; hence, the ionic radius should
decrease smoothly with an increase in atomic number.