Page 213 - MODERN ELECTROCHEMISTRY
P. 213
ION–SOLVENT INTERACTIONS 149
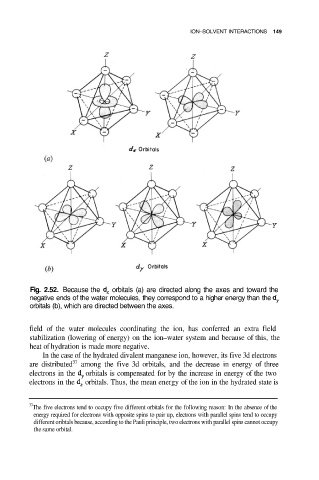
Fig. 2.52. Because the orbitals (a) are directed along the axes and toward the
negative ends of the water molecules, they correspond to a higher energy than the
orbitals (b), which are directed between the axes.
field of the water molecules coordinating the ion, has conferred an extra field
stabilization (lowering of energy) on the ion–water system and because of this, the
heat of hydration is made more negative.
In the case of the hydrated divalent manganese ion, however, its five 3d electrons
37
are distributed among the five 3d orbitals, and the decrease in energy of three
electrons in the orbitals is compensated for by the increase in energy of the two
electrons in the orbitals. Thus, the mean energy of the ion in the hydrated state is
37
The five electrons tend to occupy five different orbitals for the following reason: In the absence of the
energy required for electrons with opposite spins to pair up, electrons with parallel spins tend to occupy
different oribtals because, according to the Pauli principle, two electrons with parallel spins cannot occupy
the same orbital.