Page 215 - MODERN ELECTROCHEMISTRY
P. 215
ION–SOLVENT INTERACTIONS 151
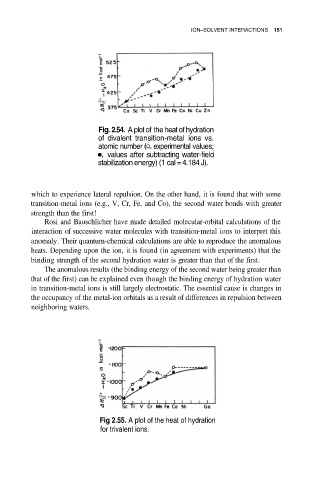
Fig. 2.54. A plot of the heat of hydration
of divalent transition-metal ions vs.
atomic number experimental values;
values after subtracting water-field
stabilization energy) (1 cal = 4.184 J).
which to experience lateral repulsion. On the other hand, it is found that with some
transition-metal ions (e.g., V, Cr, Fe, and Co), the second water bonds with greater
strength than the first!
Rosi and Bauschlicher have made detailed molecular-orbital calculations of the
interaction of successive water molecules with transition-metal ions to interpret this
anomaly. Their quantum-chemical calculations are able to reproduce the anomalous
heats. Depending upon the ion, it is found (in agreement with experiments) that the
binding strength of the second hydration water is greater than that of the first.
The anomalous results (the binding energy of the second water being greater than
that of the first) can be explained even though the binding energy of hydration water
in transition-metal ions is still largely electrostatic. The essential cause is changes in
the occupancy of the metal-ion orbitals as a result of differences in repulsion between
neighboring waters.
Fig 2.55. A plot of the heat of hydration
for trivalent ions.