Page 211 - MODERN ELECTROCHEMISTRY
P. 211
ION–SOLVENT INTERACTIONS 147
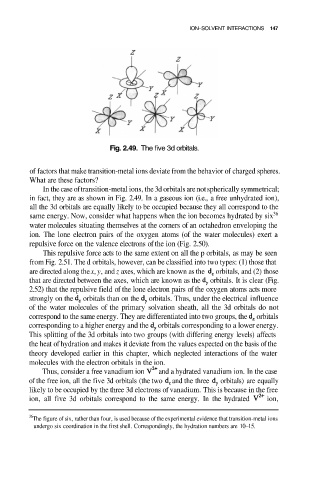
Fig. 2.49. The five 3d orbitals.
of factors that make transition-metal ions deviate from the behavior of charged spheres.
What are these factors?
In the case of transition-metal ions, the 3d orbitals are not spherically symmetrical;
in fact, they are as shown in Fig. 2.49. In a gaseous ion (i.e., a free unhydrated ion),
all the 3d orbitals are equally likely to be occupied because they all correspond to the
same energy. Now, consider what happens when the ion becomes hydrated by six 36
water molecules situating themselves at the corners of an octahedron enveloping the
ion. The lone electron pairs of the oxygen atoms (of the water molecules) exert a
repulsive force on the valence electrons of the ion (Fig. 2.50).
This repulsive force acts to the same extent on all the p orbitals, as may be seen
from Fig. 2.51. The d orbitals, however, can be classified into two types: (1) those that
are directed along the x, y, and z axes, which are known as the orbitals, and (2) those
that are directed between the axes, which are known as the orbitals. It is clear (Fig.
2.52) that the repulsive field of the lone electron pairs of the oxygen atoms acts more
strongly on the orbitals than on the orbitals. Thus, under the electrical influence
of the water molecules of the primary solvation sheath, all the 3d orbitals do not
correspond to the same energy. They are differentiated into two groups, the orbitals
corresponding to a higher energy and the orbitals corresponding to a lower energy.
This splitting of the 3d orbitals into two groups (with differing energy levels) affects
the heat of hydration and makes it deviate from the values expected on the basis of the
theory developed earlier in this chapter, which neglected interactions of the water
molecules with the electron orbitals in the ion.
Thus, consider a free vanadium ion and a hydrated vanadium ion. In the case
of the free ion, all the five 3d orbitals (the two and the three orbitals) are equally
likely to be occupied by the three 3d electrons of vanadium. This is because in the free
ion, all five 3d orbitals correspond to the same energy. In the hydrated ion,
36
The figure of six, rather than four, is used because of the experimental evidence that transition-metal ions
undergo six coordination in the first shell. Correspondingly, the hydration numbers are 10–15.