Page 209 - MODERN ELECTROCHEMISTRY
P. 209
ION–SOLVENT INTERACTIONS 145
activity, entropy, mobility, partial ionic volume, vibration potentials, and dielectric
constant). There seem to be two interpretations for this marked discrepancy. The first
is that the spectroscopic methods are relatively insensitive and for this reason are only
applicable to concentrated solutions. However, here the number of water molecules
available per ion markedly decreases water molecules in a M solution
and 10 water molecules in a 5 M solution) so that there would be a mass tendency
toward lower hydration numbers (see Fig. 2.46). Apart from this, in solutions as
concentrated as those used, e.g., in neutron diffraction for 2:1 salts
such as the situation becomes complicated for two reasons: (1) the formation
of various kinds of ion pairs and triplets and (2) the fact that so much of the water
available is part of the hydration sheaths that are the object of investigation. Thus, ionic
concentration, which refers conceptually to the number of free waters in which the
hydrated ion can move, has a different meaning from that when the number of water
molecules that are tied up is negligible.
Conversely, spectroscopic methods (particularly NMR and neutron diffraction)
can be used to sense the residence time of the water molecules within the solvent
sheaths around the ion. Thus, they could offer the most important data still required—a
clean quantitative determination of the number of molecules that move with the ion.
Unfortunately they only work in concentration regions far higher than those of the
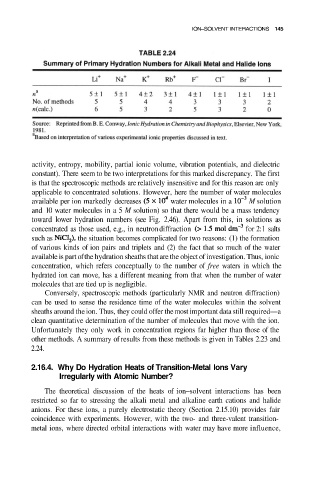
other methods. A summary of results from these methods is given in Tables 2.23 and
2.24.
2.16.4. Why Do Hydration Heats of Transition-Metal Ions Vary
Irregularly with Atomic Number?
The theoretical discussion of the heats of ion–solvent interactions has been
restricted so far to stressing the alkali metal and alkaline earth cations and halide
anions. For these ions, a purely electrostatic theory (Section 2.15.10) provides fair
coincidence with experiments. However, with the two- and three-valent transition-
metal ions, where directed orbital interactions with water may have more influence,