Page 212 - MODERN ELECTROCHEMISTRY
P. 212
148 CHAPTER 2
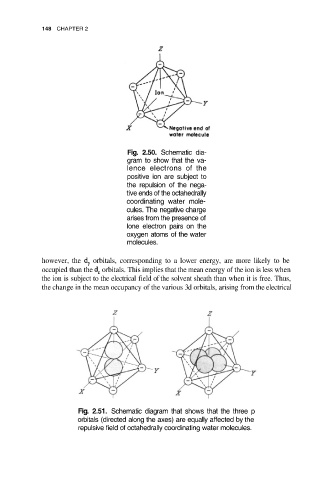
Fig. 2.50. Schematic dia-
gram to show that the va-
lence electrons of the
positive ion are subject to
the repulsion of the nega-
tive ends of the octahedrally
coordinating water mole-
cules. The negative charge
arises from the presence of
lone electron pairs on the
oxygen atoms of the water
molecules.
however, the orbitals, corresponding to a lower energy, are more likely to be
occupied than the orbitals. This implies that the mean energy of the ion is less when
the ion is subject to the electrical field of the solvent sheath than when it is free. Thus,
the change in the mean occupancy of the various 3d orbitals, arising from the electrical
Fig. 2.51. Schematic diagram that shows that the three p
orbitals (directed along the axes) are equally affected by the
repulsive field of octahedrally coordinating water molecules.