Page 202 - MODERN ELECTROCHEMISTRY
P. 202
ION–SOLVENT INTERACTIONS 139
2.15.15. Krestov’s Separation of Ion and Solvent Effects in Ion
Hydration
Krestov in 1993 broke down the solvent contribution into an “ordering” one—that
is, a negative entropy due to the enhanced order brought about by the ordering of the
solvent around the ion—and a disordering entropy (i.e., positive) caused by the
solvent breakdown. Temperature affects the ordering part little, but the disordering
contribution diminishes with an increase in temperature because the water is already
broken up before entry of the ion.
2.16. MORE ON SOLVATION NUMBERS
2.16.1. Introduction
One of the challenges of solvation studies consists in separating effects among
the ions of a salt (e.g., those due to the anion and those due to the cation) and this
difficulty, that of determining the individual solvation heats (see Section 2.15), invades
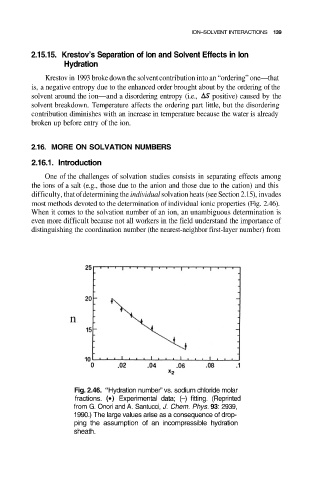
most methods devoted to the determination of individual ionic properties (Fig. 2.46).
When it comes to the solvation number of an ion, an unambiguous determination is
even more difficult because not all workers in the field understand the importance of
distinguishing the coordination number (the nearest-neighbor first-layer number) from
Fig. 2.46. “Hydration number” vs. sodium chloride molar
fractions. Experimental data; (–) fitting. (Reprinted
from G. Onori and A. Santucci, J. Chem. Phys. 93: 2939,
1990.) The large values arise as a consequence of drop-
ping the assumption of an incompressible hydration
sheath.