Page 222 - MODERN ELECTROCHEMISTRY
P. 222
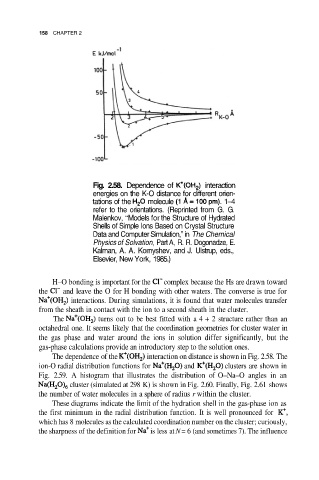
158 CHAPTER 2
Fig. 2.58. Dependence of interaction
energies on the K-O distance for different orien-
tations of the molecule 1–4
refer to the orientations. (Reprinted from G. G.
Malenkov, “Models for the Structure of Hydrated
Shells of Simple Ions Based on Crystal Structure
Data and Computer Simulation,” in The Chemical
Physics of Solvation, Part A, R. R. Dogonadze, E.
Kalman, A. A. Kornyshev, and J. Ulstrup, eds.,
Elsevier, New York, 1985.)
H–O bonding is important for the complex because the Hs are drawn toward
the and leave the O for H bonding with other waters. The converse is true for
interactions. During simulations, it is found that water molecules transfer
from the sheath in contact with the ion to a second sheath in the cluster.
The turns out to be best fitted with a 4 + 2 structure rather than an
octahedral one. It seems likely that the coordination geometries for cluster water in
the gas phase and water around the ions in solution differ significantly, but the
gas-phase calculations provide an introductory step to the solution ones.
The dependence of the interaction on distance is shown in Fig. 2.58. The
ion-O radial distribution functions for and clusters are shown in
Fig. 2.59. A histogram that illustrates the distribution of O–Na–O angles in an
cluster (simulated at 298 K) is shown in Fig. 2.60. Finally, Fig. 2.61 shows
the number of water molecules in a sphere of radius r within the cluster.
These diagrams indicate the limit of the hydration shell in the gas-phase ion as
the first minimum in the radial distribution function. It is well pronounced for
which has 8 molecules as the calculated coordination number on the cluster; curiously,
the sharpness of the definition for is less at N = 6 (and sometimes 7). The influence