Page 224 - MODERN ELECTROCHEMISTRY
P. 224
160 CHAPTER 2
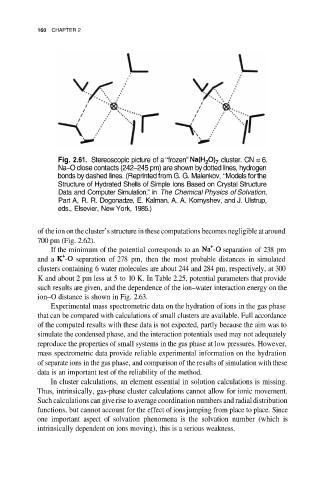
Fig. 2.61. Stereoscopic picture of a “frozen” cluster. CN = 6.
Na–O close contacts (242–245 pm) are shown by dotted lines, hydrogen
bonds by dashed lines. (Reprinted from G. G. Malenkov, “Models for the
Structure of Hydrated Shells of Simple Ions Based on Crystal Structure
Data and Computer Simulation,” in The Chemical Physics of Solvation,
Part A, R. R. Dogonadze, E. Kalman, A. A. Kornyshev, and J. Ulstrup,
eds., Elsevier, New York, 1985.)
of the ion on the cluster’s structure in these computations becomes negligible at around
700 pm (Fig. 2.62).
If the minimum of the potential corresponds to an separation of 238 pm
and a separation of 278 pm, then the most probable distances in simulated
clusters containing 6 water molecules are about 244 and 284 pm, respectively, at 300
K and about 2 pm less at 5 to 10 K. In Table 2.25, potential parameters that provide
such results are given, and the dependence of the ion–water interaction energy on the
ion–O distance is shown in Fig. 2.63.
Experimental mass spectrometric data on the hydration of ions in the gas phase
that can be compared with calculations of small clusters are available. Full accordance
of the computed results with these data is not expected, partly because the aim was to
simulate the condensed phase, and the interaction potentials used may not adequately
reproduce the properties of small systems in the gas phase at low pressures. However,
mass spectrometric data provide reliable experimental information on the hydration
of separate ions in the gas phase, and comparison of the results of simulation with these
data is an important test of the reliability of the method.
In cluster calculations, an element essential in solution calculations is missing.
Thus, intrinsically, gas-phase cluster calculations cannot allow for ionic movement.
Such calculations can give rise to average coordination numbers and radial distribution
functions, but cannot account for the effect of ions jumping from place to place. Since
one important aspect of solvation phenomena is the solvation number (which is
intrinsically dependent on ions moving), this is a serious weakness.