Page 223 - MODERN ELECTROCHEMISTRY
P. 223
ION–SOLVENT INTERACTIONS 159
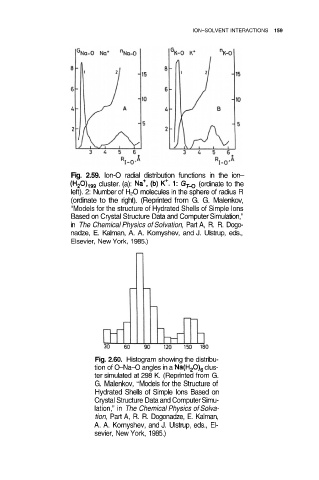
Fig. 2.59. lon-O radial distribution functions in the ion–
cluster. (a): (ordinate to the
left). 2: Number of H 2O molecules in the sphere of radius R
(ordinate to the right). (Reprinted from G. G. Malenkov,
“Models for the structure of Hydrated Shells of Simple Ions
Based on Crystal Structure Data and Computer Simulation,”
in The Chemical Physics of Solvation, Part A, R. R. Dogo-
nadze, E. Kalman, A. A. Kornyshev, and J. Ulstrup, eds.,
Elsevier, New York, 1985.)
Fig. 2.60. Histogram showing the distribu-
tion of O–Na–O angles in a clus-
ter simulated at 298 K. (Reprinted from G.
G. Malenkov, “Models for the Structure of
Hydrated Shells of Simple Ions Based on
Crystal Structure Data and Computer Simu-
lation,” in The Chemical Physics of Solva-
tion, Part A, R. R. Dogonadze, E. Kalman,
A. A. Kornyshev, and J. Ulstrup, eds., El-
sevier, New York, 1985.)