Page 225 - Modern physical chemistry
P. 225
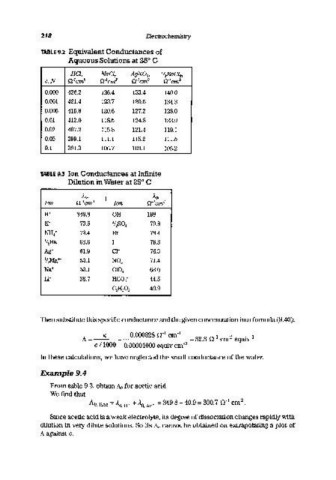
218 Electrochemistry
TABLE 9.2 Equivalent Conductances of
Aqueous Solutions at 25° C
HCl, NaCl, AgNOI» l/:eBaCl2>
c,N n- 1cm 2 n- 1cm 2 n- 1cm 2 n- 1cm 2
0.000 426.2 126.4 133.4 140.0
0.001 421.4 123.7 130.5 134.3
0.005 415.8 120.6 127.2 128.0
0.01 412.0 118.5 124.8 123.9
0.02 407.2 115.8 121.4 119.1
0.05 399.1 111.1 115.2 111.5
0.1 391.3 106.7 109.1 105.2
TABLE 9.3 Ion Conductances at Infinite
Dilution in Water at 25° C
AD> AD>
Ion n- 1cm 2 Ion n- 1cm 2
H+ 349.8 OH- 198
K+ 73.5 1/2S0t 79.8
NH 4 + 73.4 Br- 78.4
1/2Ba++ 63.6 1- 76.8
Ag+ 61.9 Cl- 76.3
1/2Mg++ 53.1 N0 3 - 71.4
Na+ 50.1 CI0 4 - 68.0
Li+ 38.7 HC0 3 - 44.5
C 2 H 3 0 2 - 40.9
Then substitute this specific conductance and the given concentration into formula (9.40):
1
·-1
0.000828 n- cm -1
2
82 8 n.-l
A = 1C --------= . ~~ cm equlv
c /1000 0.00001000 equiv cm-3
In these calculations, we have neglected the small conductance of the water.
Example 9.4
From table 9.3, obtain 1\0 for acetic acid.
We find that
2
1
Ao, HAc = 1.. 0 , W + 1.. 0 , Ac- = 349.8 + 40.9 = 390.7 n- cm •
Since acetic acid is a weak electrolyte, its degree of dissociation changes rapidly with
dilution in very dilute solutions. So its 1\0 cannot be obtained on extrapolating a plot of
A against c.