Page 17 - Multidimensional Chromatography
P. 17
6 Multidimensional Chromatography
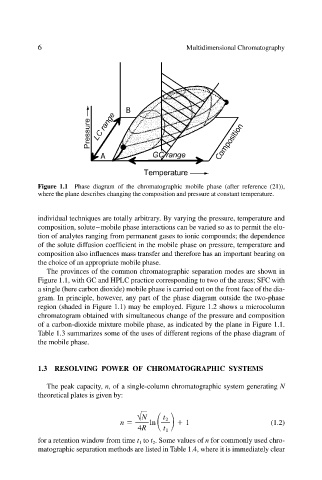
Figure 1.1 Phase diagram of the chromatographic mobile phase (after reference (21)),
where the plane describes changing the composition and pressure at constant temperature.
individual techniques are totally arbitrary. By varying the pressure, temperature and
composition, solute–mobile phase interactions can be varied so as to permit the elu-
tion of analytes ranging from permanent gases to ionic compounds; the dependence
of the solute diffusion coefficient in the mobile phase on pressure, temperature and
composition also influences mass transfer and therefore has an important bearing on
the choice of an appropriate mobile phase.
The provinces of the common chromatographic separation modes are shown in
Figure 1.1, with GC and HPLC practice corresponding to two of the areas; SFC with
a single (here carbon dioxide) mobile phase is carried out on the front face of the dia-
gram. In principle, however, any part of the phase diagram outside the two-phase
region (shaded in Figure 1.1) may be employed. Figure 1.2 shows a microcolumn
chromatogram obtained with simultaneous change of the pressure and composition
of a carbon-dioxide mixture mobile phase, as indicated by the plane in Figure 1.1.
Table 1.3 summarizes some of the uses of different regions of the phase diagram of
the mobile phase.
1.3 RESOLVING POWER OF CHROMATOGRAPHIC SYSTEMS
The peak capacity, n, of a single-column chromatographic system generating N
theoretical plates is given by:
√N t 2
n ln 1 (1.2)
4R t 1
for a retention window from time t 1 to t 2 . Some values of n for commonly used chro-
matographic separation methods are listed in Table 1.4, where it is immediately clear