Page 184 - Multifunctional Photocatalytic Materials for Energy
P. 184
170 Multifunctional Photocatalytic Materials for Energy
8.4 Semiconductor nanomaterials as mesoporous
layers for DSSCs
In this section, we focus on uses for different metal oxide semiconductors (TiO 2 , ZnO,
and SnO 2 ) as photoanodes in DSSCs, with an expectation that highly efficient and
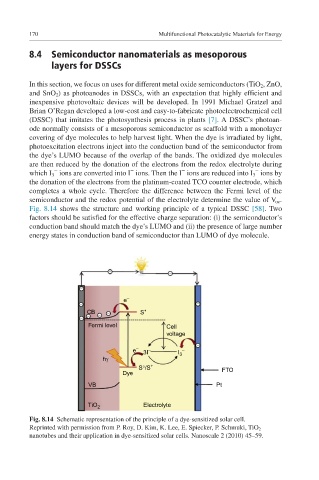
inexpensive photovoltaic devices will be developed. In 1991 Michael Gratzel and
Brian O’Regan developed a low-cost and easy-to-fabricate photoelectrochemical cell
(DSSC) that imitates the photosynthesis process in plants [7]. A DSSC’s photoan-
ode normally consists of a mesoporous semiconductor as scaffold with a monolayer
covering of dye molecules to help harvest light. When the dye is irradiated by light,
photoexcitation electrons inject into the conduction band of the semiconductor from
the dye’s LUMO because of the overlap of the bands. The oxidized dye molecules
are then reduced by the donation of the electrons from the redox electrolyte during
−
−
−
−
which I 3 ions are converted into I ions. Then the I ions are reduced into I 3 ions by
the donation of the electrons from the platinum-coated TCO counter electrode, which
completes a whole cycle. Therefore the difference between the Fermi level of the
semiconductor and the redox potential of the electrolyte determine the value of V oc .
Fig. 8.14 shows the structure and working principle of a typical DSSC [58]. Two
factors should be satisfied for the effective charge separation: (i) the semiconductor’s
conduction band should match the dye’s LUMO and (ii) the presence of large number
energy states in conduction band of semiconductor than LUMO of dye molecule.
− −
−
e –
− −
CB − S *
− − −
Fermi level Cell
voltage
−
e – 3I – I 3 –
hg
S°/S + FTO
Dye
VB Pt
TiO 2 Electrolyte
Fig. 8.14 Schematic representation of the principle of a dye-sensitized solar cell.
Reprinted with permission from P. Roy, D. Kim, K. Lee, E. Spiecker, P. Schmuki, TiO 2
nanotubes and their application in dye-sensitized solar cells. Nanoscale 2 (2010) 45–59.