Page 208 - Multifunctional Photocatalytic Materials for Energy
P. 208
Metal-based semiconductor nanomaterials for photocatalysis 193
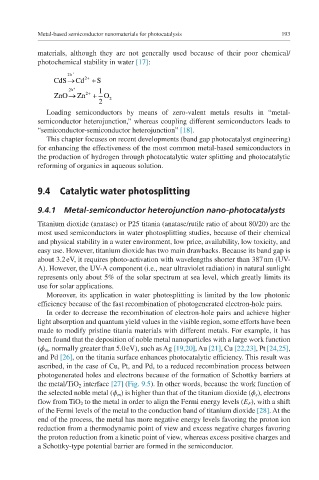
materials, although they are not generally used because of their poor chemical/
photochemical stability in water [17]:
2h +
CdS® Cd 2 + + S
2 h + 1
ZnO® Zn 2 + + O
2 2
Loading semiconductors by means of zero-valent metals results in “metal-
semiconductor heterojunction,” whereas coupling different semiconductors leads to
“semiconductor-semiconductor heterojunction” [18].
This chapter focuses on recent developments (band gap photocatalyst engineering)
for enhancing the effectiveness of the most common metal-based semiconductors in
the production of hydrogen through photocatalytic water splitting and photocatalytic
reforming of organics in aqueous solution.
9.4 Catalytic water photosplitting
9.4.1 Metal-semiconductor heterojunction nano-photocatalysts
Titanium dioxide (anatase) or P25 titania (anatase/rutile ratio of about 80/20) are the
most used semiconductors in water photosplitting studies, because of their chemical
and physical stability in a water environment, low price, availability, low toxicity, and
easy use. However, titanium dioxide has two main drawbacks. Because its band gap is
about 3.2 eV, it requires photo-activation with wavelengths shorter than 387 nm (UV-
A). However, the UV-A component (i.e., near ultraviolet radiation) in natural sunlight
represents only about 5% of the solar spectrum at sea level, which greatly limits its
use for solar applications.
Moreover, its application in water photosplitting is limited by the low photonic
efficiency because of the fast recombination of photogenerated electron-hole pairs.
In order to decrease the recombination of electron-hole pairs and achieve higher
light absorption and quantum yield values in the visible region, some efforts have been
made to modify pristine titania materials with different metals. For example, it has
been found that the deposition of noble metal nanoparticles with a large work function
(ϕ m , normally greater than 5.0 eV), such as Ag [19,20], Au [21], Cu [22,23], Pt [24,25],
and Pd [26], on the titania surface enhances photocatalytic efficiency. This result was
ascribed, in the case of Cu, Pt, and Pd, to a reduced recombination process between
photogenerated holes and electrons because of the formation of Schottky barriers at
the metal/TiO 2 interface [27] (Fig. 9.5). In other words, because the work function of
the selected noble metal (ϕ m ) is higher than that of the titanium dioxide (ϕ s ), electrons
flow from TiO 2 to the metal in order to align the Fermi energy levels (E F ), with a shift
of the Fermi levels of the metal to the conduction band of titanium dioxide [28]. At the
end of the process, the metal has more negative energy levels favoring the proton ion
reduction from a thermodynamic point of view and excess negative charges favoring
the proton reduction from a kinetic point of view, whereas excess positive charges and
a Schottky-type potential barrier are formed in the semiconductor.