Page 213 - Multifunctional Photocatalytic Materials for Energy
P. 213
198 Multifunctional Photocatalytic Materials for Energy
Comprehensive lists of mixed semiconductor heterostructures for water photosplit-
ting are reported by others [76,77], whereas the band gap values for a large number of
semiconductors are reported by Luque and Balu [78].
The highest specific rates of hydrogen production are reached over Pt-decorated
4
Cu 1.94 S-Zn 0.23 Cd 0.77 S (>1.3⋅10 μmol H 2 /h⋅gr [61]), Na,SrTiO 3 -Rh x Cr 2−x O 3
4
4
(>2.2⋅10 μmol H 2 /h⋅gr [79]), and NiO-NaTaO 3 (>2.9⋅10 μmol H 2 /h⋅gr [76]) un-
der UV irradiation (λ > 300 nm). Under visible light radiation, the best specific pro-
5
ductivities are obtained over CdS/ZnS (2.4⋅10 μmol H 2 /h⋅gr [62], ZnS-In 2 S 3 -CuS
5
5
(3.6⋅10 μmol H 2 /h⋅gr [80]), and ZnFe 2 O 4 -SrTiO 3 (>4.0⋅10 μmol H 2 /h⋅gr [81]).
In addition to traditional oxide photocatalysts, new complex nanocomposites based
on metal sulfides, metal oxysulfides, tantalates, nitrides, and oxynitrides have been
investigated. In several studies, water photosplitting was achieved by adopting a com-
bination of photocatalysts for H 2 (i.e., doped SrTiO 3 ) and O 2 (i.e., BiVO 4 ) generation.
Although coupling n-p type semiconductors can reduce an electron-hole recombi-
nation, and because the recombination cannot be totally removed, hydrogen and ox-
ygen production is difficult to achieve. Therefore selected inorganic ions, such as
3+
4+
− −
2−
2−
S /SO 3 [82], IO 3 /I [83], and Ce /Ce [84], are used in order to increase the lifetime
of photogenerated charges, and in some cases to prevent the photocorrosion of metal
sulfides used as n- or p-type semiconductors. These species generally act as redox me-
diators for the n-p type semiconductor pair [17]. The species in the lower oxidation state
scavenges the holes generated in one of the two semiconductors; photogenerated elec-
trons are thus free to reduce protons to form hydrogen. On the contrary, the species in the
higher oxidation state reacts with electrons of the second semiconductors, thus favoring
the oxidation reaction between photogenerated holes and water to generate oxygen [85].
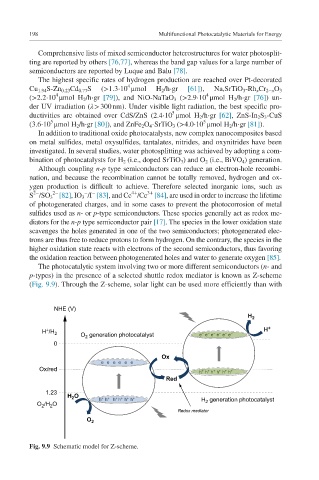
The photocatalytic system involving two or more different semiconductors (n- and
p-types) in the presence of a selected shuttle redox mediator is known as Z-scheme
(Fig. 9.9). Through the Z-scheme, solar light can be used more efficiently than with
NHE (V)
H 2
+
H /H 2 O generation photocatalyst e e e e e e − H +
−
−
−
−
−
2
0
Ox
−
−
−
−
−
e e e e e e −
Ox/red + + + + + +
h h h h h h
Red
1.23
H O h h + h h h h + generation photocatalyst
+
2
+
+
+
O 2 /H 2 O H 2
Redox mediator
O 2
Fig. 9.9 Schematic model for Z-scheme.