Page 209 - Multifunctional Photocatalytic Materials for Energy
P. 209
194 Multifunctional Photocatalytic Materials for Energy
(A) (B)
E vacuum
E
f s vacuum
f m E cb f m
E F e − e −
E F e − V D
E F e − e − E cb
E vb
Metal
Semiconductor Accumulation region Depletion region
E vb
Metal Semiconductor
Heterojunction
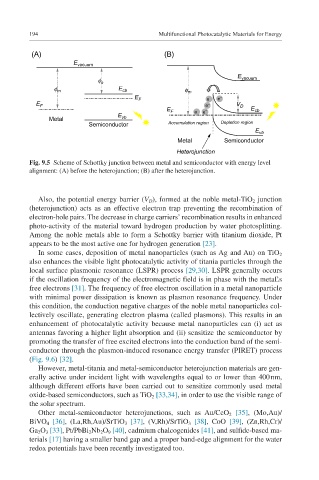
Fig. 9.5 Scheme of Schottky junction between metal and semiconductor with energy level
alignment: (A) before the heterojunction; (B) after the heterojunction.
Also, the potential energy barrier (V D ), formed at the noble metal-TiO 2 junction
(heterojunction) acts as an effective electron trap preventing the recombination of
electron-hole pairs. The decrease in charge carriers’ recombination results in enhanced
photo-activity of the material toward hydrogen production by water photosplitting.
Among the noble metals able to form a Schottky barrier with titanium dioxide, Pt
appears to be the most active one for hydrogen generation [23].
In some cases, deposition of metal nanoparticles (such as Ag and Au) on TiO 2
also enhances the visible light photocatalytic activity of titania particles through the
local surface plasmonic resonance (LSPR) process [29,30]. LSPR generally occurs
if the oscillation frequency of the electromagnetic field is in phase with the metaĽs
free electrons [31]. The frequency of free electron oscillation in a metal nanoparticle
with minimal power dissipation is known as plasmon resonance frequency. Under
this condition, the conduction negative charges of the noble metal nanoparticles col-
lectively oscillate, generating electron plasma (called plasmons). This results in an
enhancement of photocatalytic activity because metal nanoparticles can (i) act as
antennas favoring a higher light absorption and (ii) sensitize the semiconductor by
promoting the transfer of free excited electrons into the conduction band of the semi-
conductor through the plasmon-induced resonance energy transfer (PIRET) process
(Fig. 9.6) [32].
However, metal-titania and metal-semiconductor heterojunction materials are gen-
erally active under incident light with wavelengths equal to or lower than 400 nm,
although different efforts have been carried out to sensitize commonly used metal
oxide-based semiconductors, such as TiO 2 [33,34], in order to use the visible range of
the solar spectrum.
Other metal-semiconductor heterojunctions, such as Au/CeO 2 [35], (Mo,Au)/
BiVO 4 [36], (La,Rh,Au)/SrTiO 3 [37], (V,Rh)/SrTiO 3 [38], CoO [39], (Zn,Rh,Cr)/
Ga 2 O 3 [33], Pt/PbBi 2 Nb 2 O 9 [40], cadmium chalcogenides [41], and sulfide-based ma-
terials [17] having a smaller band gap and a proper band-edge alignment for the water
redox potentials have been recently investigated too.