Page 234 - Multifunctional Photocatalytic Materials for Energy
P. 234
Photocatalysts for hydrogen generation and organic contaminants degradation 217
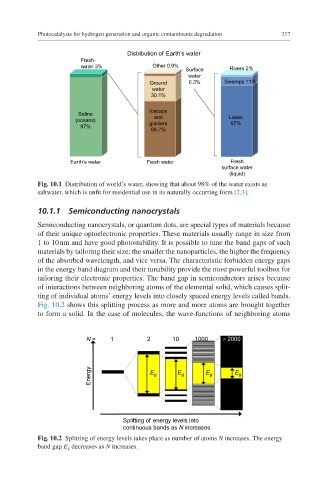
Distribution of Earth’s water
Fresh-
water 3% Other 0.9%
Surface Rivers 2%
water
Ground 0.3% Swamps 11%
water
30.1%
Icecaps
Saline and Lakes
(oceans) glaciers 87%
97%
68.7%
Earth’s water Fresh water Fresh
surface water
(liquid)
Fig. 10.1 Distribution of world’s water, showing that about 98% of the water exists as
saltwater, which is unfit for residential use in its naturally occurring form [2,3].
10.1.1 Semiconducting nanocrystals
Semiconducting nanocrystals, or quantum dots, are special types of materials because
of their unique optoelectronic properties. These materials usually range in size from
1 to 10 nm and have good photostability. It is possible to tune the band gaps of such
materials by tailoring their size; the smaller the nanoparticles, the higher the frequency
of the absorbed wavelength, and vice versa. The characteristic forbidden energy gaps
in the energy band diagram and their tunability provide the most powerful toolbox for
tailoring their electronic properties. The band gap in semiconductors arises because
of interactions between neighboring atoms of the elemental solid, which causes split-
ting of individual atoms’ energy levels into closely spaced energy levels called bands.
Fig. 10.2 shows this splitting process as more and more atoms are brought together
to form a solid. In the case of molecules, the wave-functions of neighboring atoms
N = 1 2 10 1000 > 2000
Energy E g E g E g E g
Splitting of energy levels into
continuous bands as N increases
Fig. 10.2 Splitting of energy levels takes place as number of atoms N increases. The energy
band gap E g decreases as N increases.