Page 91 - Multifunctional Photocatalytic Materials for Energy
P. 91
80 Multifunctional Photocatalytic Materials for Energy
photoreduction of CO 2 . The chapter is organized into three major sections: (i) a brief
introduction of graphene and its most common derivatives; (ii) a review of several
important synthesis strategies for graphene-based photocatalysts; and (iii) the applica-
tion of graphene-based semiconductor photocatalysts in photocatalytic water splitting
to H 2 and photocatalytic reduction of CO 2 to hydrocarbon fuels. Finally, the major
challenges for the future development of graphene-based photocatalysts to produce
solar fuels are identified.
5.2 Graphene and its derivatives
The first isolation of graphene was obtained simply by mechanical exfoliation of
graphite using the Scotch tape method [10]. However, this method of preparation is
not suitable for assembling graphene with other materials and for large-scale produc-
tion. Many feasible routes have been developed to prepare various types of graphene
materials, such as chemical vapor deposition (CVD), epitaxial growth, and so on
[17,18]. However, the most popular methods available to produce graphene-based
materials involve an initial strong chemical oxidation of natural graphite to graphite
oxide, followed by its mechanical, chemical, or thermal exfoliation to GO sheets,
which then can be chemically or thermally reduced, resulting in the rGO material
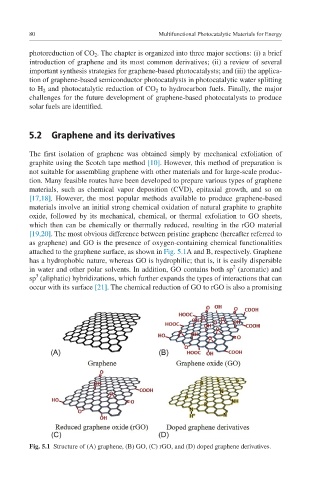
[19,20]. The most obvious difference between pristine graphene (hereafter referred to
as graphene) and GO is the presence of oxygen-containing chemical functionalities
attached to the graphene surface, as shown in Fig. 5.1A and B, respectively. Graphene
has a hydrophobic nature, whereas GO is hydrophilic; that is, it is easily dispersible
2
in water and other polar solvents. In addition, GO contains both sp (aromatic) and
3
sp (aliphatic) hybridizations, which further expands the types of interactions that can
occur with its surface [21]. The chemical reduction of GO to rGO is also a promising
Fig. 5.1 Structure of (A) graphene, (B) GO, (C) rGO, and (D) doped graphene derivatives.