Page 151 - Book Hosokawa Nanoparticle Technology Handbook
P. 151
3.4 ADSORPTION PROPERTIES AND WETTABILITY OF NANOPARTICLE SURFACE FUNDAMENTALS
of physical chemistry, the former is physical adsorp-
tion and the latter is chemical adsorption.
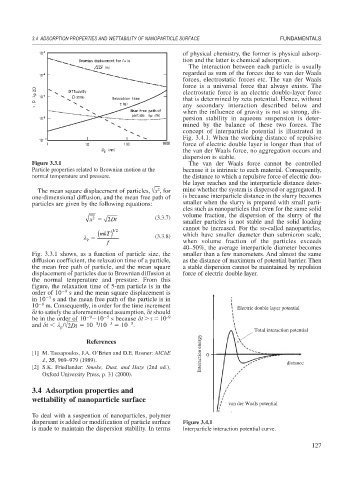
The interaction between each particle is usually
regarded as sum of the forces due to van der Waals
forces, electrostatic forces etc. The van der Waals
force is a universal force that always exists. The
electrostatic force is an electric double-layer force
that is determined by zeta potential. Hence, without
any secondary interaction described below and
when the influence of gravity is not so strong, dis-
persion stability in aqueous suspension is deter-
mined by the balance of these two forces. The
concept of interparticle potential is illustrated in
Fig. 3.4.1. When the working distance of repulsive
force of electric double layer is longer than that of
the van der Waals force, no aggregation occurs and
dispersion is stable.
Figure 3.3.1 The van der Waals force cannot be controlled
Particle properties related to Brownian motion at the because it is intrinsic to each material. Consequently,
normal temperature and pressure. the distance to which a repulsive force of electric dou-
ble layer reaches and the interparticle distance deter-
2
The mean square displacement of particles, x , for mine whether the system is dispersed or aggregated. It
one-dimensional diffusion, and the mean free path of is because interparticle distance in the slurry becomes
particles are given by the following equations: smaller when the slurry is prepared with small parti-
cles such as nanoparticles that even for the same solid
volume fraction, the dispersion of the slurry of the
x 2 Dt (3.3.7)
2
smaller particles is not stable and the solid loading
( mkT) 12 cannot be increased. For the so-called nanoparticles,
(3.3.8) which have smaller diameter than submicron scale,
P
f when volume fraction of the particles exceeds
40–50%, the average interparticle diameter becomes
Fig. 3.3.1 shows, as a function of particle size, the smaller than a few nanometers. And almost the same
diffusion coefficient, the relaxation time of a particle, as the distance of maximum of potential barrier. Then
the mean free path of particle, and the mean square a stable dispersion cannot be maintained by repulsion
displacement of particles due to Brownian diffusion at force of electric double layer.
the normal temperature and pressure. From this
figure, the relaxation time of 5-nm particle is in the
order of 10 9 s and the mean square displacement is
in 10 3 s and the mean free path of the particle is in
10 8 m. Consequently, in order for the time increment Electric double layer potential
t to satisfy the aforementioned assumption, t should
9
be in the order of 10 10 5 s because t 10 –9
5
8
and t / 2Dt 10 /10 3 10 .
p
Total interaction potential
Interaction energy 0
References
[1] M. Tassapoulos, J.A. O’Brien and D.E. Rosner: AIChE
J., 35, 969–979 (1989). distance
[2] S.K. Friedlander: Smoke, Dust, and Haze (2nd ed.),
Oxford University Press, p. 31 (2000).
3.4 Adsorption properties and
wettability of nanoparticle surface
van der Waals potential
To deal with a suspention of nanoparticles, polymer
dispersant is added or modification of particle surface Figure 3.4.1
is made to maintain the dispersion stability. In terms Interparticle interaction potential curve.
127