Page 152 - Book Hosokawa Nanoparticle Technology Handbook
P. 152
FUNDAMENTALS CH. 3 CHARACTERISTICS AND BEHAVIOR OF NANOPARTICLES AND ITS DISPERSION SYSTEMS
Therefore, the particle distance in the slurry at a Here, taking polymeric dispersant as an example,
high solid loading of nanoparticles is small and the relationship between interacting particles and
particles aggregate easily. Physical adsorption of adsorbing behavior of dispersant to nanoparticles in
polymeric dispersant or surface modification using aqueous system has been described. It is important to
chemical adsorption is effective in order to disperse notice that the effects arise from adsorption of dis-
the particles at such high concentration. Adsorbed persant depend on the surface area or weight of the
polymeric dispersants increase the interparticle dis- particles. Proper usage and selection of dispersant
tance by steric repulsion and the dissociation of mod- can be derived from aforementioned concepts in the
ifier molecules increases electrostatic repulsion force. cases of non-aqueous slurry or surface modification
From these reasons above, dispersants or modifiers by chemical reaction.
with steric adsorption structure that keeps the inter- It is ideal that dispersion is achieved during particle
particle distance and dissociates to increase electro- synthesis process in liquid phase to prepare nanopar-
static repulsion should be selected. ticle suspension. However, in many cases, raw materi-
The selection of polymeric dispersant considering als for chemical industry are supplied as dry powder.
the properties and structure of adsorbing material is To prepare stable nanoparticle dispersion from these
introduced below. It is said that polymeric dispersant dry materials, affinity between a solvent and a surface
of the molecular weight about 10,000 effectively of particle (wettability) is important.
stabilize the dispersion. Dispersants of excess molec- Contact angle measurement is one of the methods
ular weight sometimes promote aggregation by inter- to evaluate the wettability of a particle. For large par-
particle bridging effect. ticles, contact angle can be measured directly using
Less amount of dispersant than the optimum con- microscope [3]. The contact angle is also obtained
centration in which the dispersant perfectly covers the from force curve of a particle attached on the probe of
surface of the particles, causes collision of the parti- atomic force microscopy [4], although the contact
cles and bridging with the polymer, and for excess angle of nanoparticles cannot be measured directly.
amount, bridging causes aggregation of the particles. Therefore, a contact angle of nanoparticles is cal-
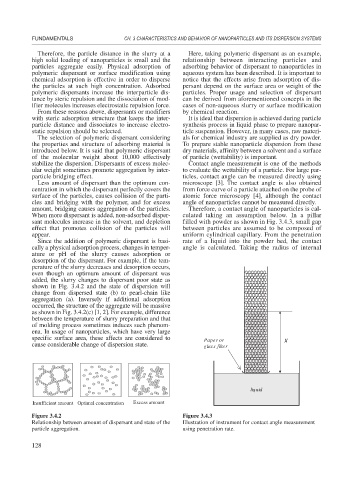
When more dispersant is added, non-adsorbed disper- culated taking an assumption below. In a pillar
sant molecules increase in the solvent, and depletion filled with powder as shown in Fig. 3.4.3, small gap
effect that promotes collision of the particles will between particles are assumed to be composed of
appear. uniform cylindrical capillary. From the penetration
Since the addition of polymeric dispersant is basi- rate of a liquid into the powder bed, the contact
cally a physical adsorption process, changes in temper- angle is calculated. Taking the radius of internal
ature or pH of the slurry causes adsorption or
desorption of the dispersant. For example, if the tem-
perature of the slurry decreases and desorption occurs,
even though an optimum amount of dispersant was
added, the slurry changes to dispersant poor state as
shown in Fig. 3.4.2 and the state of dispersion will
change from dispersed state (b) to pearl-chain like
aggregation (a). Inversely if additional adsorption
occurred, the structure of the aggregate will be massive
as shown in Fig. 3.4.2(c) [1, 2]. For example, difference
between the temperature of slurry preparation and that
of molding process sometimes induces such phenom-
ena. In usage of nanoparticles, which have very large
specific surface area, these affects are considered to Paper or X
cause considerable change of dispersion state. glass filter
liquid
Insufficient amount Optimal concentration Excess amount
Figure 3.4.2 Figure 3.4.3
Relationship between amount of dispersant and state of the Illustration of instrument for contact angle measurement
particle aggregation. using penetration rate.
128