Page 156 - Book Hosokawa Nanoparticle Technology Handbook
P. 156
FUNDAMENTALS CH. 3 CHARACTERISTICS AND BEHAVIOR OF NANOPARTICLES AND ITS DISPERSION SYSTEMS
The relationship between the relative humidity and
the capillary condensation is represented by the fol-
α lowing Kelvin equation:
D p
2 Section
D p sin a =(D /2) sin p M
⎛ 1 1 ⎞
2 Liquid i p ln RT ⎝ ⎜ r ⎠ ⎟ (3.5.17)
bridge p s L r 2 1
r r 1
1 θ r 2
where p is the vapor pressure of the capillary con-
densed liquid, p the saturated vapor pressure of the
s
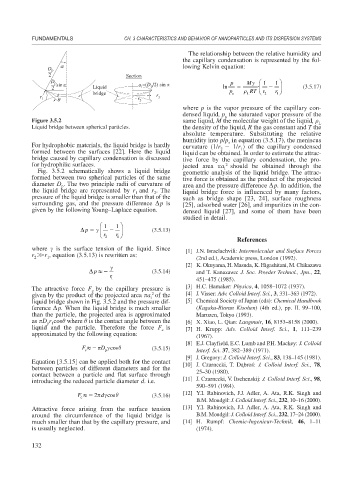
Figure 3.5.2 same liquid, M the molecular weight of the liquid, L
Liquid bridge between spherical particles. the density of the liquid, R the gas constant and T the
absolute temperature. Substituting the relative
humidity into p/p in equation (3.5.17), the meniscus
s
For hydrophobic materials, the liquid bridge is hardly curvature (1/r 1/r ) of the capillary condensed
1
2
formed between the surfaces [22]. Here the liquid liquid can be obtained. In order to estimate the attrac-
bridge caused by capillary condensation is discussed tive force by the capillary condensation, the pro-
for hydrophilic surfaces. jected area a should be obtained through the
2
i
Fig. 3.5.2 schematically shows a liquid bridge geometric analysis of the liquid bridge. The attrac-
formed between two spherical particles of the same tive force is obtained as the product of the projected
diameter D . The two principle radii of curvature of area and the pressure difference p. In addition, the
p
the liquid bridge are represented by r and r . The liquid bridge force is influenced by many factors,
1
2
pressure of the liquid bridge is smaller than that of the such as bridge shape [23, 24], surface roughness
surrounding gas, and the pressure difference p is [25], adsorbed water [26], and impurities in the con-
given by the following Young–Laplace equation. densed liquid [27], and some of them have been
studied in detail.
⎛ 1 1 ⎞
p
⎜ ⎝ r r ⎠ ⎟ (3.5.13)
2
1
References
where
is the surface tension of the liquid. Since [1] J.N. Israelachvili: Intermolecular and Surface Forces
r r , equation (3.5.13) is rewritten as: (2nd ed.), Academic press, London (1992).
2
1
[2] K. Okuyama, H. Masuda, K. Higashitani, M. Chikazawa
p
(3.5.14) and T. Kanazawa: J. Soc. Powder Technol., Jpn., 22,
r 1 451–475 (1985).
The attractive force F by the capillary pressure is [3] H.C. Hamaker: Physica, 4, 1058–1072 (1937).
c
2
given by the product of the projected area a of the [4] J. Visser: Adv. Colloid Interf. Sci., 3, 331–363 (1972).
i
liquid bridge shown in Fig. 3.5.2 and the pressure dif- [5] Chemical Society of Japan (eds): Chemical Handbook
ference p. When the liquid bridge is much smaller (Kagaku-Binran Kisohen) (4th ed.), pp. II. 99–100,
than the particle, the projected area is approximated Maruzen, Tokyo (1993).
as D r cos where is the contact angle between the [6] X. Xiao, L. Qian: Langmuir, 16, 8153–8158 (2000).
p 1
liquid and the particle. Therefore the force F is [7] H. Krupp: Adv. Colloid Interf. Sci., 1, 111–239
c
approximated by the following equation: (1967).
[8] E.J. Clayfield, E.C. Lumb and P.H. Mackey: J. Colloid
F D
cos (3.5.15) Interf. Sci. 37, 382–389 (1971).
p
c
[9] J. Gregory: J. Colloid Interf. Sci., 83, 138–145 (1981).
Equation [3.5.15] can be applied both for the contact [10] J. Czarnecki, T. Da bros´: J. Colloid Interf. Sci., 78,
between particles of different diameters and for the 25–30 (1980).
contact between a particle and flat surface through
introducing the reduced particle diameter d. i.e. [11] J. Czarnecki, V. Itschenskij: J. Colloid Interf. Sci., 98,
590–591 (1984).
F 2 d
cos (3.5.16) [12] Y.I. Rabinovich, J.J. Adler, A. Ata, R.K. Singh and
c
B.M. Moudgil: J. Colloid Interf. Sci., 232, 10–16 (2000).
Attractive force arising from the surface tension [13] Y.I. Rabinovich, J.J. Adler, A. Ata, R.K. Singh and
around the circumference of the liquid bridge is B.M. Moudgil: J. Colloid Interf. Sci., 232, 17–24 (2000).
much smaller than that by the capillary pressure, and [14] H. Rumpf: Chemie-Ingenieur-Technik, 46, 1–11
is usually neglected. (1974).
132