Page 192 - Book Hosokawa Nanoparticle Technology Handbook
P. 192
FUNDAMENTALS CH. 3 CHARACTERISTICS AND BEHAVIOR OF NANOPARTICLES AND ITS DISPERSION SYSTEMS
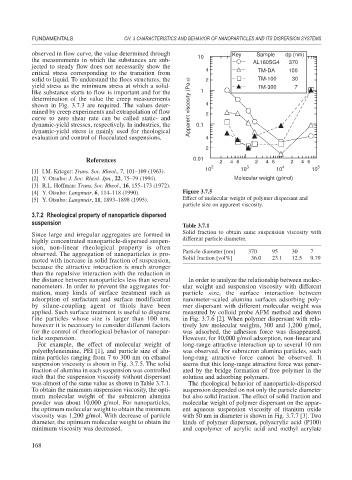
observed in flow curve, the value determined through 10 Key Sample dp (nm)
the measurements in which the substances are sub- AL160SG4 370
jected to steady flow does not necessarily show the 4
critical stress corresponding to the transition from TM-DA 100
solid to liquid. To understand the flocs structures, the 2 TM-100 30
yield stress as the minimum stress at which a solid- TM-300 7
like substance starts to flow is important and for the 1
determination of the value the creep measurements
shown in Fig. 3.7.3 are required. The values deter- Apparent viscosity (Pa⋅s) 4
mined by creep experiments and extrapolation of flow 2
curve to zero shear rate can be called static- and
dynamic-yield stresses, respectively. In industries, the 0.1
dynamic-yield stress is mainly used for rheological
evaluation and control of flocculated suspensions. 4
2
References 0.01 2 4 6 2 4 6 2 4 6
2 3 4 5
[1] I.M. Krieger: Trans. Soc. Rheol., 7, 101–109 (1963). 10 10 10 10
[2] Y. Otsubo: J. Soc. Rheol. Jpn., 22, 75–79 (1994). Molecular weight (g/mol)
[3] R.L. Hoffman: Trans. Soc. Rheol., 16, 155–173 (1972).
[4] Y. Otsubo: Langmuir, 6, 114–118 (1990). Figure 3.7.5
[5] Y. Otsubo: Langmuir, 11, 1893–1898 (1995). Effect of molecular weight of polymer dispersant and
particle size on apparent viscosity.
3.7.2 Rheological property of nanoparticle dispersed
suspension
Table 3.7.1
Since large and irregular aggregates are formed in Solid fraction to obtain same suspension viscosity with
highly concentrated nanoparticle-dispersed suspen- different particle diameter.
sion, non-linear rheological property is often
observed. The aggregation of nanoparticles is pro- Particle diameter [nm] 370 95 30 7
moted with increase in solid fraction of suspension, Solid fraction [vol%] 36.0 23.1 12.5 9.79
because the attractive interaction is much stronger
than the repulsive interaction with the reduction in
the distance between nanoparticles less than several In order to analyze the relationship between molec-
nanometers. In order to prevent the aggregates for- ular weight and suspension viscosity with different
mation, many kinds of surface treatment such as particle size, the surface interaction between
adsorption of surfactant and surface modification nanometer-scaled alumina surfaces adsorbing poly-
by silane-coupling agent or thiols have been mer dispersant with different molecular weight was
applied. Such surface treatment is useful to disperse measured by colloid probe AFM method and shown
fine particles whose size is larger than 100 nm, in Fig. 3.7.6 [2]. When polymer dispersant with rela-
however it is necessary to consider different factors tively low molecular weights, 300 and 1,200 g/mol,
for the control of rheorlogical behavior of nanopar- was adsorbed, the adhesion force was disappeared.
ticle suspension. However, for 10,000 g/mol adsorption, non-linear and
For example, the effect of molecular weight of long-range attractive interaction up to several 10 nm
polyethyleneimine, PEI [1], and particle size of alu- was observed. For submicron alumina particles, such
mina particles ranging from 7 to 300 nm on ethanol long-rang attractive force cannot be observed. It
suspension viscosity is shown in Fig. 3.7.5. The solid seems that this long-range attractive force was gener-
fraction of alumina in each suspension was controlled ated by the bridge formation of free polymer in the
such that the suspension viscosity without dispersant solution and adsorbing polymers.
was almost of the same value as shown in Table 3.7.1. The rheological behavior of nanoparticle-dispersed
To obtain the minimum suspension viscosity, the opti- suspension depended on not only the particle diameter
mum molecular weight of the submicron alumina but also solid fraction. The effect of solid fraction and
powder was about 10,000 g/mol. For nanoparticles, molecular weight of polymer dispersant on the appar-
the optimum molecular weight to obtain the minimum ent aqueous suspension viscosity of titanium oxide
viscosity was 1,200 g/mol. With decrease of particle with 50 nm in diameter is shown in Fig. 3.7.7 [3]. Two
diameter, the optimum molecular weight to obtain the kinds of polymer dispersant, polyacrylic acid (P100)
minimum viscosity was decreased. and copolymer of acrylic acid and methyl acrylate
168