Page 188 - Book Hosokawa Nanoparticle Technology Handbook
P. 188
FUNDAMENTALS CH. 3 CHARACTERISTICS AND BEHAVIOR OF NANOPARTICLES AND ITS DISPERSION SYSTEMS
ones like dielectric constant and magnetic susceptibil- fundamental structure is shown in Fig. 3.6.10(b). The
ity, the crystal phase control is important. For optical silane-coupling agent has a long history, and applied
materials, the refractive indexes of anatase phase (2.5) to various fields, for example, silica filler dispersed in
and rutile phase (2.7) are much higher than that of the insulator resin for a semiconductor and water
amorphous titanium oxide. If the crystal phase can be repellency processing of the glass. The molecular
controlled during the synthesis process of composite, structure and weight of hydrocarbon chain in both
it is needed to prevent the crystal growth of inorganic surface modification agents are designed for the pur-
particles less than several tens of nanometers. For pose of treatment.
these reasons, commercial-level products of the poly- The reaction process of both agents is shown in
mer composite have been developed only for high Fig. 3.6.10. The SH group in thiols reacts with metal
heat-resistance materials of amorphous silica com- surface directly. The –OCH or –OC H group in
2
5
3
posite. silane-coupling agents is firstly hydrolyzed with
water molecule and condensed with OH groups at the
2) Dispersion of synthesized nanoparticles into organic
surface of oxide particles. If the ideal reaction control
solvent and polymer resin is achieved, OH groups on the oxide particles are
In order to disperse inorganic particles into organic replaced by hydrocarbon chain of the coupling agent.
solvent and polymer resin, hydrophobic group and Nanometer-scale surface coating is a different
hydrocarbon chain were formed on the surface of par- approach for surface modification of nanoparticles.
ticles by various surface treatment methods. Since it The surface of metal/inorganic compound except
is difficult to modify hydrocarbon chain on the sur- metal oxide nanoparticles experiences oxidation or
face of metal or metal oxide directly, silane-coupling hydrolysis reaction in air or water. Since it is difficult
agents and thiols have been used to modify the parti- to prevent such oxidation only by the surface modifi-
cle surface of metal and metal oxide. Thiols were used cation at the molecular level, nanometer-scale surface
for surface modification of metal compounds and coating is carried out on the surface of nanoparticles.
semiconductors such as Au, Ag, CdS, and CdSe, etc. One example is silica layer coating by the hydration
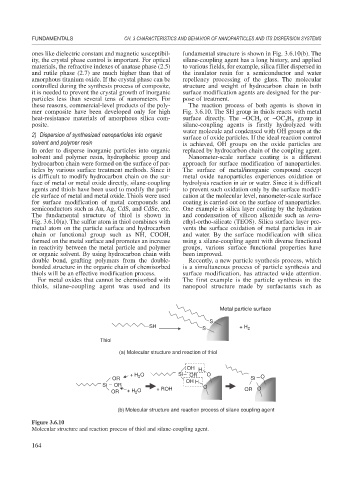
The fundamental structure of thiol is shown in and condensation of silicon alkoxide such as tetra-
Fig. 3.6.10(a). The sulfur atom in thiol combines with ethyl-ortho-silicate (TEOS). Silica surface layer pre-
metal atom on the particle surface and hydrocarbon vents the surface oxidation of metal particles in air
chain or functional group such as NH, COOH, and water. By the surface modification with silica
formed on the metal surface and promotes an increase using a silane-coupling agent with diverse functional
in reactivity between the metal particle and polymer groups, various surface functional properties have
or organic solvent. By using hydrocarbon chain with been improved.
double bond, grafting polymers from the double- Recently, a new particle synthesis process, which
bonded structure in the organic chain of chemisorbed is a simultaneous process of particle synthesis and
thiols will be an effective modification process. surface modification, has attracted wide attention.
For metal oxides that cannot be chemisorbed with The first example is the particle synthesis in the
thiols, silane-coupling agent was used and its nanopool structure made by surfactants such as
Metal particle surface
S
SH + H
S 2
Thiol
(a) Molecular structure and reaction of thiol
OH H
O Si O
+ H 2 OR O
OR Si
OH H
Si OR O
OR + H O + ROH OR O
2
(b) Molecular structure and reaction process of silane coupling agent
Figure 3.6.10
Molecular structure and reaction process of thiol and silane-coupling agent.
164