Page 184 - Book Hosokawa Nanoparticle Technology Handbook
P. 184
FUNDAMENTALS CH. 3 CHARACTERISTICS AND BEHAVIOR OF NANOPARTICLES AND ITS DISPERSION SYSTEMS
the rotating pins impinge against the feed slurry intro- 80
duced from the inlet located at the bottom of the mill.
The impingement breaks aggregates in the slurry 60
coming up through the mill. The slurry and the beads
are separated by centrifugation in the upper part of the
mill and only the slurry is discharged through the hol- 40
low shaft to the outside of the mill.
Usually, the separated slurry is circulated to a feed ζ potential / mV 20
vessel for repetition of the process. The beads less
than 100 m are effective for breaking and dispersing 0
the agglomerated nanoparticles [1–3]. Successful dis-
persion of nano-sized titanium oxide in liquid with 2 3 4 5 6 7 8 9 10
beads of 30- m diameter has been reported [2]. -20
(2) Colloidal method
-40
Fine particles repeatedly collide with each other by pH
their thermal motion and van der Waals force arising
among themselves. As a result, they agglomerate Figure 3.6.6
unless sufficient repulsion force arises. For stable Relation between pH and zeta potential (alumina).
interaction, interparticle potential should be suffi-
ciently large, while the agglomerating speed should
be extremely slow. The concept of stabilization by
electrostatic repulsion potential is well-known as SiO layer on the particle surface and NH , which
2
3
DLVO theory [4]. increases pH of the slurry up to about 10.5. Since the
The combination of the attractive van der Waals isoelectric point of SiO is around pH 2, high surface
2
interaction and the repulsive double-layer repulsion is electric potential is generated on the surface of the
the foundation of the well-known DLVO theory [4], fine particles. This self-dispersion allows the forma-
which provides an overall net interaction energy. This tion of silicon nitride water slurry at about 35 vol%
theory is successful in describing the interactions concentration [6].
between charged particles immersed in water. At a
low surface potential or a high ionic strength, the (3) Evaluation of surface electric potential
repulsive barrier vanishes, allowing particles to floc- Measurement of zeta potential is one of the methods
culate. For highly charged surfaces, there is a strong to evaluate surface electric potential in liquid, like
long-range repulsion, and the energy barrier is too electrophoresis, streaming potential, and ultrasonic
high for the particles to overcome during any reason- methods [7]. Today, excellent devices based on these
able time period. When dispersing metal oxide parti- methods are commercially available domestically and
cles in water, the net charge is controlled by pH. The abroad. Zeta potential is a little lower than surface
built-up of a charge at the solid–liquid interface is potential, however, is often used instead of the latter
resulted from proton-transfer reaction with the sur- technically.
face hydroxyl groups. Care must be taken in evaluating zeta potential,
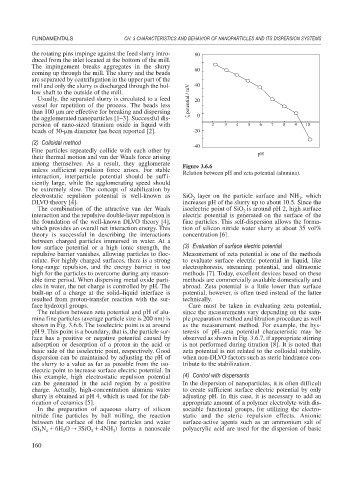
The relation between zeta potential and pH of alu- since the measurements vary depending on the sam-
mina fine particles (average particle size is 200 nm) is ple preparation method and titration procedure as well
shown in Fig. 3.6.6. The isoelectric point is at around as the measurement method. For example, the hys-
pH 9. This point is a boundary, that is, the particle sur- teresis of pH–zeta potential characteristic may be
face has a positive or negative potential caused by observed as shown in Fig. 3.6.7, if appropriate stirring
adsorption or desorption of a proton in the acid or is not performed during titration [8]. It is noted that
basic side of the isoelectric point, respectively. Good zeta potential is not related to the colloidal stability,
dispersion can be maintained by adjusting the pH of when non-DLVO factors such as steric hindrance con-
the slurry to a value as far as possible from the iso- tribute to the stabilization.
electric point to increase surface electric potential. In
this example, high electrostatic repulsion potential (4) Control with dispersants
can be generated in the acid region by a positive In the dispersion of nanoparticles, it is often difficult
charge. Actually, high-concentration alumina water to create sufficient surface electric potential by only
slurry is obtained at pH 4, which is used for the fab- adjusting pH. In this case, it is necessary to add an
rication of ceramics [5]. appropriate amount of a polymer electrolyte with dis-
In the preparation of aqueous slurry of silicon sociable functional groups, for utilizing the electro-
nitride fine particles by ball milling, the reaction static and the steric repulsion effects. Anionic
between the surface of the fine particles and water surface-active agents such as an ammonium salt of
(Si N 6H O 3SiO 4NH ) forms a nanoscale polyacrylic acid are used for the dispersion of basic
4
3
2
2
3
160