Page 181 - Book Hosokawa Nanoparticle Technology Handbook
P. 181
3.6 AGGREGATION AND DISPERSION, CHARACTERIZATION AND CONTROL FUNDAMENTALS
3.6 Aggregation and dispersion, value ranging from 10 to 50 nm in diameter, and
increases with increasing of particle density. The
characterization and control
aggregation rate in gas phase is much higher than that
in liquid since it increases with decreasing solvent
3.6.1 Aggregation and dispersion in gas phase viscosity and density. Because of high aggregation
rate, it is difficult to control the aggregation and dis-
Aggregation phenomena of nanoparticles in gas persion of nanoparticles in gas phase.
phase are generated by the collision and adhesion The aggregation phenomena of particles depend
between particles, and controlled by the Brownian on various parameters, for example, surface interac-
motion, drag force, surface interaction such as van der tion such as electrostatic and van der Waals forces
Waals and electrostatic forces, etc. Based on the fun- [2], velocity gradient and turbulent flow of gas [3],
damentals of aggregation of nanoparticles in gas acoustic wave and ultrasonic wave, and wave motion
phase, the characterization and control methods of such as microwave [4]. By using several types of
nanoparticles will be introduced. external force and interaction, the control of aggre-
gation and dispersion of nanoparticles has been
(1) Aggregation and dispersion mechanisms in gas phase studied.
The decreasing rate, dn/dt, of number base density of
uniform spherical particle with a diameter, D , dis- (2) Characterization of aggregation and dispersion
p
persed to the primary particle at the initial stage, is Many kinds of the characterization method of particle
described by the following equation; aggregation behavior in gas phase have been investi-
gated. One traditional indirect and macroscopic
dn dt K n 2 (3.6.1) method is Carr’s dispersibility determined by the
0 equipment as shown in Fig. 3.6.2 [5]. Powder with a
mass, m charged from the upper side of a cylindrical
0
where K is an aggregation rate constant which is a tube is collected on a disk with 4 inches in diameter,
0
function of particle diameter and aggregation mecha- which is placed at 24 inches lower from the cylinder
nism. If the aggregation of primary particles is con- bottom. By measuring the weight m of the collected
f
trolled only by the Brownian motion and collision of powder on the disk, the dispersion behavior of powder
particles without interaction between particles, the is characterized. For dispersed powder, since the
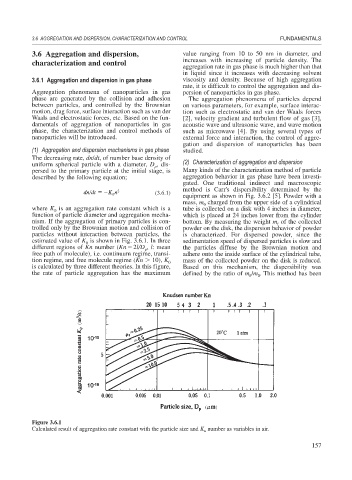
estimated value of K is shown in Fig. 3.6.1. In three sedimentation speed of dispersed particles is slow and
0
different regions of Kn number (Kn 2l/D , l: mean the particles diffuse by the Brownian motion and
p
free path of molecule), i.e. continuum regime, transi- adhere onto the inside surface of the cylindrical tube,
tion regime, and free molecule regime (Kn 10), K 0 mass of the collected powder on the disk is reduced.
is calculated by three different theories. In this figure, Based on this mechanism, the dispersibility was
the rate of particle aggregation has the maximum defined by the ratio of m /m . This method has been
f
0
Figure 3.6.1
Calculated result of aggregation rate constant with the particle size and K number as variables in air.
n
157