Page 186 - Book Hosokawa Nanoparticle Technology Handbook
P. 186
FUNDAMENTALS CH. 3 CHARACTERISTICS AND BEHAVIOR OF NANOPARTICLES AND ITS DISPERSION SYSTEMS
300 example, desorption of the dispersant at a higher tem-
Apparent viscosity (mPas at 200-s) 200 slurry. Accordingly, the temperature must be carefully
perature brings about the condition where the disper-
sant is insufficient in aggregating the particles, even if
250
an appropriate quantity of the dispersant is added to the
controlled in the fabrication processes of ceramics.
The rheological properties shown in Fig. 3.6.9 are
150
widely used for evaluating the dispersion/aggrega-
tion behavior of high concentrated slurry systems. In
100
addition, other properties are available for the evalu-
keep the shear rate constant and sedimentation,
50
deformation, and compaction properties under
mechanical fields [13]. The AMF colloid probe
0 ation. This includes shear stress change with time to
0 0.5 1 1.5 2 2.5 technique is used in liquid for evaluating micro-
Amount of dispersing agent (mass%) scopic interaction between the particles (Refer to
Section 3.5.3). It is also attempted to observe the
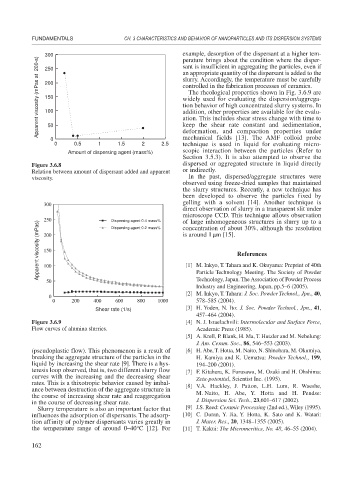
Figure 3.6.8 dispersed or aggregated structure in liquid directly
Relation between amount of dispersant added and apparent or indirectly.
viscosity. In the past, dispersed/aggregate structures were
observed using freeze-dried samples that maintained
the slurry structures. Recently, a new technique has
been developed to observe the particles fixed by
gelling with a solvent [14]. Another technique is
300
direct observation of slurry in a transparent slit under
microscope CCD. This technique allows observation
250 Dispersing agent 0.4 mass% of large inhomogeneous structures in slurry up to a
Apparent viscosity (mPas) 200 is around 1 m [15]. References
concentration of about 30%, although the resolution
Dispersing agent 0.2 mass%
150
[1] M. Inkyo, T. Tahara and K. Okuyama: Preprint of 40th
100
Technology, Japan. The Association of Powder Process
50 Particle Technology Meeting. The Society of Powder
Industry and Engineering, Japan, pp.5–6 (2005).
[2] M. Inkyo, T. Tahara: J. Soc. Powder Technol., Jpn., 40,
0
0 200 400 600 800 1000 578–585 (2004).
Shear rate (1/s) [3] H. Yoden, N. Ito: J. Soc. Powder Technol., Jpn., 41,
457–464 (2004).
Figure 3.6.9 [4] N. J. Israelachvili: Intermolecular and Surface Force,
Flow curves of alumina slurries. Academic Press (1985).
[5] A. Krell, P. Blank, H. Ma, T. Hutzler and M. Nebelung:
J. Am. Ceram. Soc., 86, 546–553 (2003).
(pseudoplastic flow). This phenomenon is a result of [6] H. Abe, T. Hotta, M. Naito, N. Shinohara, M. Okumiya,
breaking the aggregate structure of the particles in the H. Kamiya and K. Uematsu: Powder Technol., 199,
liquid by increasing the shear rate [9]. There is a hys- 194–200 (2001).
teresis loop observed, that is, two different slurry flow [7] F. Kitahara, K. Furusawa, M. Ozaki and H. Ohshima:
curves with the increasing and the decreasing shear Zeta-potential, Scientist Inc. (1995).
rates. This is a thixotropic behavior caused by imbal- [8] V.A. Hackley, J. Patton, L.H. Lum, R. Waeshe,
ance between destruction of the aggregate structure in M. Naito, H. Abe, Y. Hotta and H. Pendse:
the course of increasing shear rate and reaggregation
in the course of decreasing shear rate. J. Dispersion Sci. Tech., 23,601–617 (2002).
Slurry temperature is also an important factor that [9] J.S. Reed: Ceramic Processing (2nd ed.), Wiley (1995).
influences the adsorption of dispersants. The adsorp- [10] C. Duran, Y. Jia, Y. Hotta, K. Sato and K. Watari:
tion affinity of polymer dispersants varies greatly in J. Mater. Res., 20, 1348–1355 (2005).
the temperature range of around 0–40°C [12]. For [11] T. Kakui: The Micromeritics, No. 48, 46–55 (2004).
162