Page 183 - Book Hosokawa Nanoparticle Technology Handbook
P. 183
3.6 AGGREGATION AND DISPERSION, CHARACTERIZATION AND CONTROL FUNDAMENTALS
vapor deposition process for nanoparticle synthesis [7]. 3.6.2 Liquid phase
The deposited salt was dissolved during dispersion of
nanoparticles into liquid with surfactant or polymer Fine particles including nanoparticles are generally
dispersant. By the adsorption of surfactant, nanopar- supplied in the aggregated state, because they are eas-
ticles are dispersed in suspension. ily aggregated during the drying process of produc-
The packing and assembling structure control of tion and transportation due to pressure caused by
synthesized nanoparticles in the film or bulk material stacking. Dispersion of these aggregates in the liquid
are being developed by the use of special technology. phase requires the input of mechanical energy to
By Akedo et al. [8], aerosol gas containing fine parti- break them.
cles with several hundreds of nanometers was jetted Breaking the aggregates increases the surface
and collided on the surface of a substrate. By the col- area of the solid–liquid interface, which causes re-
lision of particles on the substrate, the particles were aggregation to impede the dispersion process if the
dispersed to nanoparticles and formed dense powder interface is unstable. Usually, the solid–liquid inter-
layer on the substrate. Gleiter’s group [9] prepared face is stabilized in the liquid phase by forming an
almost fully densed nanopolycrystalline by the follow- electric double layer or adsorption of the polymer
ing process. Nanometer-sized inorganic particles were around fine particles. For the dispersion of fine parti-
prepared by PVD method in high-vacuum chamber. cles in liquid, how to mechanically disperse fine par-
Prepared nanoparticles were packed into small metal ticles in liquid and control their stability are very
mold, and consolidated by one axial pressing with important together with the evaluation technique.
ultra high pressure up to 5 GPa in vacuum chamber.
Based on this work, the aggregated structure of oxide (1) Mechanical dispersion
nanoparticles in atmosphere was able to be collapsed The mechanical dispersion methods are classified
and packed to almost hexagonal closed-packing struc- into stirring, high-speed revolution shearing, milling,
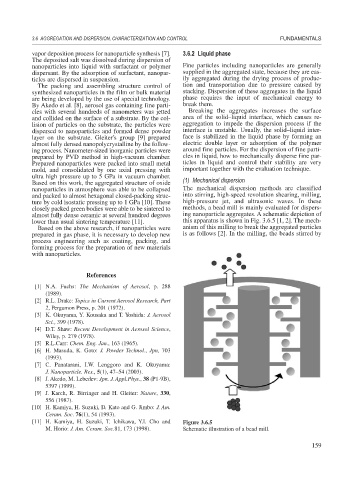
ture by cold isostatic pressing up to 1 GPa [10]. These high-pressure jet, and ultrasonic waves. In these
closely packed green bodies were able to be sintered to methods, a bead mill is mainly evaluated for dispers-
almost fully dense ceramic at several hundred degrees ing nanoparticle aggregates. A schematic depiction of
lower than usual sintering temperature [11]. this apparatus is shown in Fig. 3.6.5 [1, 2]. The mech-
Based on the above research, if nanoparticles were anism of this milling to break the aggregated particles
prepared in gas phase, it is necessary to develop new is as follows [2]. In the milling, the beads stirred by
process engineering such as coating, packing, and
forming process for the preparation of new materials
with nanoparticles.
References
[1] N.A. Fuchs: The Mechanism of Aerosol, p. 288
(1989).
[2] R.L. Drake: Topics in Current Aerosol Research, Part
2, Pergamon Press, p. 201 (1972).
[3] K. Okuyama, Y. Kousaka and T. Yoshida: J. Aerosol
Sci., 399 (1978).
[4] D.T. Shaw: Recent Development in Aerosol Science,
Wiley, p. 279 (1978).
[5] R.L.Carr: Chem. Eng. Jan., 163 (1965).
[6] H. Masuda, K. Goto: J. Powder Technol., Jpn, 703
(1993).
[7] C. Panatarani, I.W. Lenggoro and K. Okuyama:
J. Nanoparticle. Res., 5(1), 47–54 (2003).
[8] J. Akedo, M. Lebedev: Jpn. J. Appl.Phys., 38 (P1-9B),
5397 (1999).
[9] J. Karch, R. Birringer and H. Gleiter: Nature, 330,
556 (1987).
[10] H. Kamiya, H. Suzuki, D. Kato and G. Jimbo: J. Am.
Ceram. Soc. 76(1), 54 (1993).
[11] H. Kamiya, H. Suzuki, T. Ichikawa, Y.I. Cho and Figure 3.6.5
M. Horio: J. Am. Ceram. Soc.81, 173 (1998). Schematic illustration of a bead mill.
159