Page 246 - Book Hosokawa Nanoparticle Technology Handbook
P. 246
FUNDAMENTALS CH. 4 CONTROL OF NANOSTRUCTURE OF MATERIALS
(2) Nanostructure control of alloy by low temperature
synthesis
On the other hand, a suitable thermal history is nec-
essary to produce the bulky Mg Si materials via the
2
complete reaction between Mg and Si. Differential
thermal analysis (DTA) evaluation is carried out to
optimize the heating temperature of RPWed Mg-Si
compacts because Mg-Si reaction is accompanied
with an exothermic heat of 89.2±11.4 kJ/mol [5]. As
shown in Fig. 4.4.30, the originally raw Mg-Si mix-
ture without RPW (N 0) reveals the remarkably
exothermic heat at 873 K and a small endothermic at
923K due to melting of the Mg elements. With
increasing the number of RPW cycles, the starting
temperature of the exothermic reaction decreases.
That is, the solid-state synthesis of Mg Si occurs at
2
lower temperature; for example it starts at about
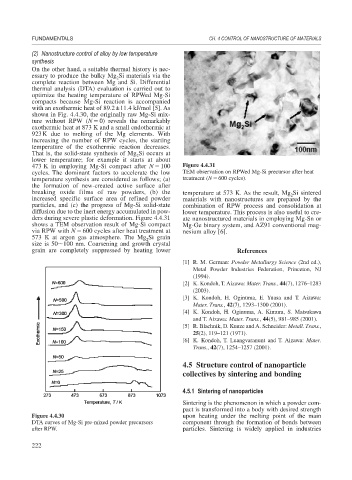
473 K in employing Mg-Si compact after N 100 Figure 4.4.31
cycles. The dominant factors to accelerate the low TEM observation on RPWed Mg-Si precursor after heat
temperature synthesis are considered as follows; (a) treatment (N 600 cycles).
the formation of new-created active surface after
breaking oxide films of raw powders, (b) the temperature at 573 K. As the result, Mg Si sintered
2
increased specific surface area of refined powder materials with nanostructures are prepared by the
particles, and (c) the progress of Mg-Si solid-state combination of RPW process and consolidation at
diffusion due to the inert energy accumulated in pow- lower temperature. This process is also useful to cre-
ders during severe plastic deformation. Figure 4.4.31 ate nanostructured materials in employing Mg-Sn or
shows a TEM observation result of Mg-Si compact Mg-Ge binary system, and AZ91 conventional mag-
via RPW with N 600 cycles after heat treatment at nesium alloy [6].
573 K at argon gas atmosphere. The Mg Si grain
2
size is 50 100 nm. Coarsening and growth crystal
grain are completely suppressed by heating lower References
[1] R. M. German: Powder Metallurgy Science (2nd ed.),
Metal Powder Industries Federation, Princeton, NJ
(1994).
[2] K. Kondoh, T. Aizawa: Mater. Trans., 44(7), 1276–1283
(2003).
[3] K. Kondoh, H. Oginuma, E. Yuasa and T. Aizawa:
Mater. Trans., 42(7), 1293–1300 (2001).
[4] K. Kondoh, H. Oginuma, A. Kimura, S. Matsukawa
and T. Aizawa: Mater. Trans., 44(5), 981–985 (2001).
[5] R. Blachnik, D. Kunze and A. Schneider: Metall. Trans.,
25(2), 119–121 (1971).
[6] K. Kondoh, T. Luangvaranunt and T. Aizawa: Mater.
Trans., 42(7), 1254–1257 (2001).
4.5 Structure control of nanoparticle
collectives by sintering and bonding
4.5.1 Sintering of nanoparticles
Sintering is the phenomenon in which a powder com-
pact is transformed into a body with desired strength
Figure 4.4.30 upon heating under the melting point of the main
DTA curves of Mg-Si pre-mixed powder precursors component through the formation of bonds between
after RPW. particles. Sintering is widely applied in industries
222