Page 273 - Book Hosokawa Nanoparticle Technology Handbook
P. 273
FUNDAMENTALS CH. 4 CONTROL OF NANOSTRUCTURE OF MATERIALS
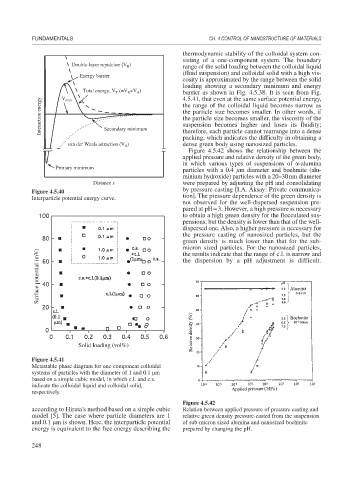
thermodynamic stability of the colloidal system con-
sisting of a one-component system. The boundary
Double-layer repulcion (V ) range of the solid loading between the colloidal liquid
R
(fluid suspension) and colloidal solid with a high vis-
Energy barrier
cosity is approximated by the range between the solid
loading showing a secondary minimum and energy
Total energy, V (=V +V ) barrier as shown in Fig. 4.5.38. It is seen from Fig.
A
R
T
V
4.5.41, that even at the same surface potential energy,
Interaction energy the range of the colloidal liquid becomes narrow as
max
the particle size becomes smaller. In other words, if
the particle size becomes smaller, the viscosity of the
suspension becomes higher and loses its fluidity;
Secondary minimum
therefore, each particle cannot rearrange into a dense
packing, which indicates the difficulty in obtaining a
van der Waals attraction (V ) dense green body using nanosized particles.
A
Figure 4.5.42 shows the relationship between the
applied pressure and relative density of the green body,
in which various types of suspensions of -alumina
Primary minimum
particles with a 0.4 m diameter and boehmite (alu-
minium hydroxide) particles with a 20–30nm diameter
Distance x were prepared by adjusting the pH and consolidating
by pressure casting [I.A. Aksay: Private communica-
Figure 4.5.40
Interparticle potential energy curve. tion]. The pressure dependence of the green density is
not observed for the well-dispersed suspension pre-
pared at pH 3. However, a high pressure is necessary
to obtain a high green density for the flocculated sus-
pensions, but the density is lower than that of the well-
dispersed one. Also, a higher pressure is necessary for
the pressure casting of nanosized particles, but the
green density is much lower than that for the sub-
micron sized particles. For the nanosized particles,
the results indicate that the range of c.l. is narrow and
the dispersion by a pH adjustment is difficult.
Figure 4.5.41
Metastable phase diagram for one-component colloidal
systems of particles with the diameter of 1 and 0.1
m
based on a simple cubic model, in which c.l. and c.s.
indicate the colloidal liquid and colloidal solid,
respectively.
Figure 4.5.42
according to Hirata’s method based on a simple cubic Relation between applied pressure of pressure casting and
model [5]. The case where particle diameters are 1 relative green density pressure-casted from the suspension
and 0.1
m is shown. Here, the interparticle potential of sub-micron sized alumina and nanosized boehmite
energy is equivalent to the free energy describing the prepared by changing the pH.
248