Page 278 - Book Hosokawa Nanoparticle Technology Handbook
P. 278
4.6 SELF-ASSEMBLY FUNDAMENTALS
Gas-liquid
Capillary force
interface
van der Waals force
nanoparticles
substrate
drying
Brownian force Particles contact force
Electrochemical force
Friction force on a substrate
Drag force from liquid
ordering
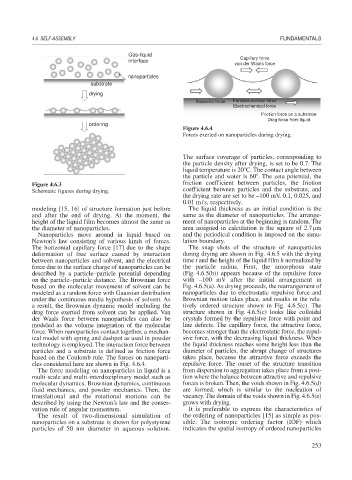
Figure 4.6.4
Forces exerted on nanoparticles during drying.
The surface coverage of particles, corresponding to
the particle density after drying, is set to be 0.7. The
liquid temperature is 20 C. The contact angle between
the particle and water is 60 . The zeta potential, the
Figure 4.6.3 friction coefficient between particles, the friction
Schematic figures during drying. coefficient between particles and the substrate, and
the drying rate are set to be –100 mV, 0.1, 0.025, and
0.01 m/s, respectively.
modeling [15, 16] of structure formation just before The liquid thickness as an initial condition is the
and after the end of drying. At the moment, the same as the diameter of nanoparticles. The arrange-
height of the liquid film becomes almost the same as ment of nanoparticles at the beginning is random. The
the diameter of nanoparticles. area assigned in calculation is the square of 2.7
m
Nanoparticles move around in liquid based on and the periodical condition is imposed on the simu-
Newton’s law consisting of various kinds of forces. lation boundary.
The horizontal capillary force [17] due to the shape The snap shots of the structure of nanoparticles
deformation of free surface caused by interaction during drying are shown in Fig. 4.6.5 with the drying
between nanoparticles and solvent, and the electrical time t and the height of the liquid film h normalized by
force due to the surface charge of nanoparticles can be the particle radius. First, the amorphous state
described by a particle–particle potential depending (Fig. 4.6.5(b)) appears because of the repulsive force
on the particle–particle distance. The Brownian force with –100 mV after the initial arrangement in
based on the molecular movement of solvent can be Fig. 4.6.5(a). As drying proceeds, the rearrangement of
modeled as a random force with Gaussian distribution nanoparticles due to electrostatic repulsive force and
under the continuous media hypothesis of solvent. As Brownian motion takes place, and results in the rela-
a result, the Brownian dynamic model including the tively ordered structure shown in Fig. 4.6.5(c). The
drag force exerted from solvent can be applied. Van structure shown in Fig. 4.6.5(c) looks like colloidal
der Waals force between nanoparticles can also be crystals formed by the repulsive force with point and
modeled as the volume integration of the molecular line defects. The capillary force, the attractive force,
force. When nanoparticles contact together, a mechan- becomes stronger than the electrostatic force, the repul-
ical model with spring and dashpot as used in powder sive force, with the decreasing liquid thickness. When
technology is employed. The interaction force between the liquid thickness reaches some height less than the
particles and a substrate is defined as friction force diameter of particles, the abrupt change of structures
based on the Coulomb rule. The forces on nanoparti- takes place, because the attractive force exceeds the
cles considered here are shown in Fig. 4.6.4. repulsive force. The onset of the structure transition
The force modeling on nanoparticles in liquid is a from dispersion to aggregation takes place from a posi-
multi-scale and multi-interdisciplinary model such as tion where the balance between attractive and repulsive
molecular dynamics, Brownian dynamics, continuous forces is broken. Then, the voids shown in Fig. 4.6.5(d)
fluid mechanics, and powder mechanics. Then, the are formed, which is similar to the nucleation of
translational and the rotational motions can be vacancy. The domain of the voids shown in Fig.4.6.5(e)
described by using the Newton’s law and the conser- grows with drying.
vation rule of angular momentum. It is preferable to express the characteristics of
The result of two-dimensional simulation of the ordering of nanoparticles [15] as simple as pos-
nanoparticles on a substrate is shown for polystyrene sible. The isotropic ordering factor (IOF) which
particles of 50 nm diameter in aqueous solution. indicates the spatial isotropy of ordered nanoparticles
253