Page 280 - Book Hosokawa Nanoparticle Technology Handbook
P. 280
4.6 SELF-ASSEMBLY FUNDAMENTALS
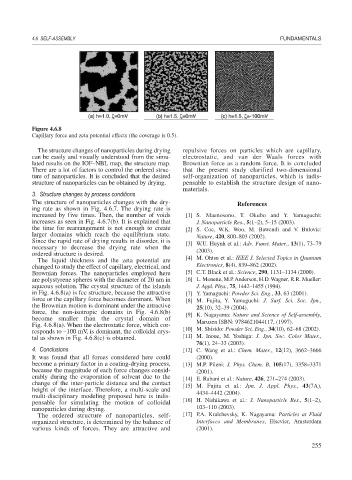
Figure 4.6.8
Capillary force and zeta potential effects (the coverage is 0.5).
The structure changes of nanoparticles during drying repulsive forces on particles which are capillary,
can be easily and visually understood from the simu- electrostatic, and van der Waals forces with
lated results on the IOF–NBL map, the structure map. Brownian force as a random force. It is concluded
There are a lot of factors to control the ordered struc- that the present study clarified two-dimensional
ture of nanoparticles. It is concluded that the desired self-organization of nanoparticles, which is indis-
structure of nanoparticles can be obtained by drying. pensable to establish the structure design of nano-
materials.
3. Structure changes by process conditions
The structure of nanoparticles changes with the dry- References
ing rate as shown in Fig. 4.6.7. The drying rate is
increased by five times. Then, the number of voids [1] S. Maenosono, T. Okubo and Y. Yamaguchi:
increases as seen in Fig. 4.6.7(b). It is explained that J. Nanoparticle Res., 5(1–2), 5–15 (2003).
the time for rearrangement is not enough to create [2] S. Coe, W.K. Woo, M. Bawendi and V. Bulovic:
larger domains which reach the equilibrium state. Nature, 420, 800–803 (2002).
Since the rapid rate of drying results in disorder, it is [3] W.U. Huynh et al.: Adv. Funct. Mater., 13(1), 73–79
necessary to decrease the drying rate when the (2003).
ordered structure is desired.
The liquid thickness and the zeta potential are [4] M. Ohtsu et al.: IEEE J. Selected Topics in Quantum
changed to study the effect of capillary, electrical, and Electronics, 8(4), 839–862 (2002).
Brownian forces. The nanoparticles employed here [5] C.T. Black et al.: Science, 290, 1131–1134 (2000).
are polystyrene spheres with the diameter of 20 nm in [6] L. Monette, M.P. Anderson, H.D. Wagner, R.R. Mueller:
aqueous solution. The crystal structure of the islands J. Appl. Phys., 75, 1442–1455 (1994).
in Fig. 4.6.8(a) is fcc structure, because the attractive [7] Y. Yamaguchi: Powder Sci. Eng., 33, 63 (2001).
force or the capillary force becomes dominant. When [8] M. Fujita, Y. Yamaguchi: J. Surf. Sci. Soc. Jpn.,
the Brownian motion is dominant under the attractive 25(10), 32–39 (2004).
force, the non-isotropic domains in Fig. 4.6.8(b) [9] K. Nagayama: Nature and Science of Self-assembly,
become smaller than the crystal domain of Maruzen ISBN: 9784621044117, (1997).
Fig. 4.6.8(a). When the electrostatic force, which cor-
responds to –100 mV, is dominant, the colloidal crys- [10] M. Shisido: Powder Sci. Eng., 34(10), 62–68 (2002).
tal as shown in Fig. 4.6.8(c) is obtained. [11] M. Inoue, M. Yoshiga: J. Jpn. Soc. Color Mater.,
76(1), 24–33 (2003).
4. Conclusions [12] C. Wang et al.: Chem. Mater., 12(12), 3662–3666
It was found that all forces considered here could (2000).
become a primary factor in a coating-drying process, [13] M.P. Pileni: J. Phys. Chem. B, 105(17), 3358–3371
because the magnitude of each force changes consid- (2001).
erably during the evaporation of solvent due to the [14] E. Rabani et al.: Nature, 426, 271–274 (2003).
change of the inter-particle distance and the contact [15] M. Fujita et al.: Jpn. J. Appl. Phys., 43(7A),
height of the interface. Therefore, a multi-scale and 4434–4442 (2004).
multi-disciplinary modeling proposed here is indis-
pensable for simulating the motion of colloidal [16] H. Nishikawa et al.: J. Nanoparticle Res., 5(1–2),
nanoparticles during drying. 103–110 (2003).
The ordered structure of nanoparticles, self- [17] P.A. Kralchevsky, K. Nagayama: Particles at Fluid
organized structure, is determined by the balance of Interfaces and Membranes, Elsevier, Amsterdam
various kinds of forces. They are attractive and (2001).
255