Page 287 - Book Hosokawa Nanoparticle Technology Handbook
P. 287
FUNDAMENTALS CH. 4 CONTROL OF NANOSTRUCTURE OF MATERIALS
[8] Y. Masuda, K. Koumoto: Nano/micro-patterning Silica
Process Technology in Next Generation, Science &
Technology Co., Ltd, Tokyo, Japan, pp. 180–188 Organic
(2006).
[9] Y. Masuda: J. Soc. Powder Technol., Jpn., 43,
362–371 (2006).
[10] Y. Masuda: Annu. Report Murata Sci. Foundation, 19,
345–355 (2005).
[11] Y. Masuda: Bull. Ceram. Soc. Jpn. 41(5), 346–351
(2006).
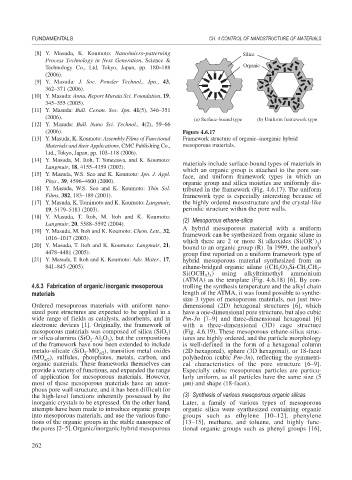
(a) Surface-bound type (b) Uniform framework type
[12] Y. Masuda: Bull. Nano Sci. Technol., 4(2), 59–66
(2006). Figure 4.6.17
[13] Y. Masuda, K. Koumoto: Assembly Films of Functional Framework structure of organic–inorganic hybrid
Materials and their Applications, CMC Publishing Co., mesoporous materials.
Ltd., Tokyo, Japan, pp. 103–118 (2006).
[14] Y. Masuda, M. Itoh, T. Yonezawa, and K. Koumoto:
materials include surface-bound types of materials in
Langmuir, 18, 4155–4159 (2002).
which an organic group is attached to the pore sur-
[15] Y. Masuda, W.S. Seo and K. Koumoto: Jpn. J. Appl.
face, and uniform framework types in which an
Phys., 39, 4596–4600 (2000). organic group and silica moieties are uniformly dis-
[16] Y. Masuda, W.S. Seo and K. Koumoto: Thin Sol. tributed in the framework (Fig. 4.6.17). The uniform
Films, 382, 183–189 (2001). framework type is especially interesting because of
[17] Y. Masuda, K. Tomimoto and K. Koumoto: Langmuir, the highly ordered mesostructure and the crystal-like
19, 5179–5183 (2003). periodic structure within the pore walls.
[18] Y. Masuda, T. Itoh, M. Itoh and K. Koumoto:
(2) Mesoporous ethane-silica
Langmuir, 20, 5588–5592 (2004).
A hybrid mesoporous material with a uniform
[19] Y. Masuda, M. Itoh and K. Koumoto: Chem. Lett., 32,
framework can be synthesized from organic silane in
1016–1017 (2003).
which there are 2 or more Si alkoxides (Si(OR’) )
[20] Y. Masuda, T. Itoh and K. Koumoto: Langmuir, 21, 3
bound to an organic group (R). In 1999, the author’s
4478–4481 (2005). group first reported on a uniform framework type of
[21] Y. Masuda, T. Itoh and K. Koumoto: Adv. Mater., 17, hybrid mesoporous material synthesized from an
841–845 (2005). ethane-bridged organic silane ((CH O) Si-CH CH -
2
2
3
3
Si(OCH ) ) using alkyltrimethyl ammonium
3 3
(ATMA) as the template (Fig. 4.6.18) [6]. By con-
4.6.3 Fabrication of organic/inorganic mesoporous trolling the synthesis temperature and the alkyl chain
materials length of the ATMA, it was found possible to synthe-
size 3 types of mesoporous materials, not just two-
Ordered mesoporous materials with uniform nano- dimensional (2D) hexagonal structures [6], which
sized pore structures are expected to be applied in a have a one-dimensional pore structure, but also cubic
wide range of fields as catalysts, adsorbents, and in Pm-3n [7–9] and three-dimensional hexagonal [6]
electronic devices [1]. Originally, the framework of with a three-dimensional (3D) cage structure
mesoporous materials was composed of silica (SiO ) (Fig. 4.6.19). These mesoporous ethane-silica struc-
2
or silica-alumina (SiO -Al O ), but the compositions tures are highly ordered, and the particle morphology
3
2
2
of the framework have now been extended to include is well-defined in the form of a hexagonal column
metalo-silicate (SiO -MO ), transition metal oxides (2D hexagonal), sphere (3D hexagonal), or 18-facet
2
n/2
(MO ), sulfides, phosphates, metals, carbon, and polyhedron (cubic Pm-3n), reflecting the symmetri-
n/2
organic materials. These frameworks themselves can cal characteristics of the pore structure [6–9].
provide a variety of functions, and expanded the range Especially cubic mesoporous particles are particu-
of application for mesoporous materials. However, larly uniform, as all particles have the same size (5
most of these mesoporous materials have an amor-
m) and shape (18-facet).
phous pore wall structure, and it has been difficult for
the high-level functions inherently possessed by the (3) Synthesis of various mesoporous organic silicas
inorganic crystals to be expressed. On the other hand, Later, a family of various types of mesoporous
attempts have been made to introduce organic groups organic silica were synthesized containing organic
into mesoporous materials, and use the various func- groups such as ethylene [10–12], phenylene
tions of the organic groups in the stable nanospace of [13–15], methane, and toluene, and highly func-
the pores [2–5].Organic/inorganic hybrid mesoporous tional organic groups such as phenyl groups [16],
262