Page 395 - Book Hosokawa Nanoparticle Technology Handbook
P. 395
6.5 ELECTROCHEMICAL PROPERTIES FUNDAMENTALS
diffusion or volume diffusion mechanism, respec- Pore size
tively. Oxygen molecule turns into oxygen ion with CGO Film distribution
the supplied electrons after arriving at the TPB and
is transported under a concentration gradation of small
oxygen between the cathode and anode thorough the
electrolyte. In the anode, oxygen ion turns back to
oxygen molecule or water or carbon dioxide depend-
ing on the kind of fuel supplied to the anode. The
released electrons cause output current of the SOFC
by connecting both electrodes thorough an outside Ice Growth Direction
load. The differences in the ionization energy of the
electrodes derived from their composition and
microstructure affect the reactivity between gas mol-
ecules and ions [5–8].
The state-of-the-art anode, Ni-YSZ cermet, fine
particles are used to produce a large number of active
TPB sites. The electrochemical active zone consists LSCF CGO Support 10μm large
of percolated matrix having ionic and electrical con-
ductors with the following advantages: (1) high Gas
porous surface area with excellent electrochemical
conductivity, (2) high electronic conductivity, Figure 6.5.19
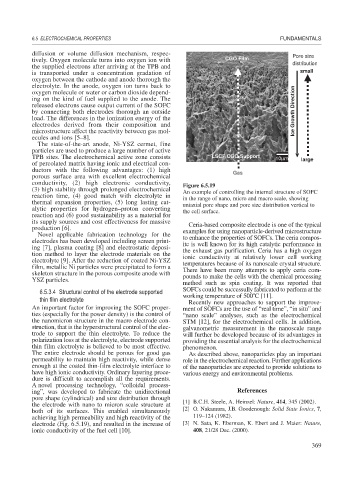
(3) high stability through prolonged electrochemical An example of controlling the internal structure of SOFC
reaction time, (4) good match with electrolyte in in the range of nano, micro and macro scale, showing
thermal expansion properties, (5) long lasting cat- uniaxial pore shape and pore size distribution vertical to
alytic properties for hydrogen–proton converting the cell surface.
reaction and (6) good sustainability as a material for
its supply sources and cost effectiveness for massive Ceria-based composite electrode is one of the typical
production [6]. examples for using nanoparticle-derived microstructure
Novel applicable fabrication technology for the
electrodes has been developed including screen print- to enhance the properties of SOFCs. The ceria compos-
ite is well known for its high catalytic performance in
ing [7], plasma coating [8] and electrostatic deposi- the exhaust gas purification. Ceria has a high oxygen
tion method to layer the electrode materials on the ionic conductivity at relatively lower cell working
electrolyte [9]. After the reduction of coated Ni-YSZ temperatures because of its nanoscale crystal structure.
film, metallic Ni particles were precipitated to form a There have been many attempts to apply ceria com-
skeleton structure in the porous composite anode with pounds to make the cells with the chemical processing
YSZ particles.
method such as spin coating. It was reported that
SOFCs could be successully fabricated to perform at the
6.5.3.4 Structural control of the electrode supported
working temperature of 500 C [11].
thin film electrolyte
Recently new approaches to support the improve-
An important factor for improving the SOFC proper- ment of SOFCs are the use of “real time”, “in situ” and
ties (especially for the power density) is the control of “nano scale” analyses, such as the electrochemical
the nanomicron structure in the macro electrode con- STM [12], for the electrochemical cells. In addition,
struction, that is the hyperstructural control of the elec- galvanometric measurement in the nanoscale range
trode to support the thin electrolyte. To reduce the will further be developed because of its advantages in
polarization loss at the electrolyte, electrode supported providing the essential analysis for the electrochemical
thin film electrolyte is believed to be most effective. phenomenon.
The entire electrode should be porous for good gas As described above, nanoparticles play an important
permeability to maintain high reactivity, while dense role in the electrochemical reaction. Further applications
enough at the coated thin-film electrolyte interface to of the nanoparticles are expected to provide solutions to
have high ionic conductivity. Ordinary layering proce- various energy and environmental problems.
dure is difficult to accomplish all the requirements.
A novel processing technology, “colloidal process-
ing”, was developed to fabricate the unidirectional References
pore shape (cylindrical) and size distribution through [1] B.C.H. Steele, A. Heinzel: Nature, 414, 345 (2002).
the electrode with nano to micron scale structure at
both of its surfaces. This enabled simultaneously [2] O. Nakamura, J.B. Goodenough: Solid State Ionics, 7,
achieving high permeability and high reactivity of the 119–124 (1982).
electrode (Fig. 6.5.19), and resulted in the increase of [3] N. Sata, K. Eberman, K. Ebert and J. Maier: Nature,
ionic conductivity of the fuel cell [10]. 408, 21/28 Dec. (2000).
369