Page 392 - Book Hosokawa Nanoparticle Technology Handbook
P. 392
FUNDAMENTALS CH. 6 EVALUATION METHODS FOR PROPERTIES OF NANOSTRUCTURED BODY
hexanol chloroform and fabricate the interfaces of matrix and nanoparti-
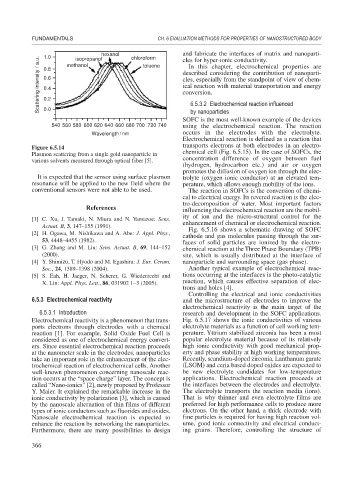
1.0
Scattering intensity / a.u. 0.6 described considering the contribution of nanoparti-
isopropanol
cles for hyper-ionic conductivity.
methanol
In this chapter, electrochemical properties are
toluene
0.8
cles, especially from the standpoint of view of chem-
ical reaction with material transportation and energy
0.4
conversion.
0.2
6.5.3.2 Electrochemical reaction influenced
0.0
by nanoparticles
SOFC is the most well-known example of the devices
540 560 580 600 620 640 660 680 700 720 740 using the electrochemical reaction. The reaction
Wavelength / nm occurs in the electrodes with the electrolyte.
Electrochemical reaction is defined as a reaction that
transports electrons at both electrodes in an electro-
Figure 6.5.14
Plasmon scattering from a single gold nanoparticle in chemical cell (Fig. 6.5.15). In the case of SOFCs, the
various solvents measured through optical fiber [5]. concentration difference of oxygen between fuel
(hydrogen, hydrocarbon etc.) and air or oxygen
promotes the diffusion of oxygen ion through the elec-
It is expected that the sensor using surface plasmon trolyte (oxygen ionic conductor) at an elevated tem-
resonance will be applied to the new field where the perature, which allows enough mobility of the ions.
conventional sensors were not able to be used. The reaction in SOFCs is the conversion of chemi-
cal to electrical energy. Its revered reaction is the elec-
tro-decomposition of water. Most important factors
References influencing the electrochemical reaction are the mobil-
ity of ion and the micro-structural control for the
[1] C. Xu, J. Tamaki, N. Miura and N. Yamazoe: Sens.
Actuat. B, 3, 147–155 (1991). enhancement of chemical or electrochemical reaction.
Fig. 6.5.16 shows a schematic drawing of SOFC
[2] H. Ogawa, M. Nishikawa and A. Abe: J. Appl. Phys., cathode and gas molecules passing through the sur-
53, 4448–4455 (1982). faces of solid particles are ionized by the electro-
[3] G. Zhang and M. Liu: Sens. Actuat. B, 69, 144–152 chemical reaction at the Three Phase Boundary (TPB)
(2000). site, which is usually distributed at the interface of
[4] Y. Shimizu, T. Hyodo and M. Egashira: J. Eur. Ceram. nanoparticle and surrounding space (gas phase).
Soc., 24, 1389–1398 (2004). Another typical example of electrochemical reac-
[5] S. Eah, H. Jaeger, N. Scherer, G. Wiederrecht and tions occurring at the interfaces is the photo-catalytic
X. Lin: Appl. Phys. Lett., 86, 031902 1–3 (2005). reaction, which causes effective separation of elec-
trons and holes [4].
Controlling the electrical and ionic conductivities
6.5.3 Electrochemical reactivity and the microstructure of electrodes to improve the
electrochemical reactivity is the main target of the
6.5.3.1 Introduction research and development in the SOFC applications.
Electrochemical reactivity is a phenomenon that trans- Fig. 6.5.17 shows the ionic conductivities of various
ports electrons through electrodes with a chemical electrolyte materials as a function of cell working tem-
reaction [1]. For example, Solid Oxide Fuel Cell is perature. Yttrium stabilized zirconia has been a most
considered as one of electrochemical energy convert- popular electrolyte material because of its relatively
ers. Since essential electrochemical reaction proceeds high ionic conductivity with good mechanical prop-
at the nanometer scale in the electrodes, nanoparticles erty and phase stability at high working temperatures.
take an important role in the enhancement of the elec- Recently, scandium-doped zirconia, Lanthanum garate
trochemical reaction of electrochemical cells. Another (LSGM) and ceria based doped oxides are expected to
well-known phenomenon concerning nanoscale reac- be new electrolyte candidates for low-temperature
tion occurs at the “space charge” layer. The concept is applications. Electrochemical reaction proceeds at
called “Nano-ionics” [2], newly proposed by Professor the interfaces between the electrodes and electrolyte.
Y. Maier. It explained the remarkable increase in the The electrolyte transports the reaction media (ions).
ionic conductivity by polarization [3], which is caused That is why thinner and even electrolyte films are
by the nanoscale alternation of thin films of different preferred for high performance cells to produce more
types of ionic conductors such as fluorides and oxides. electrons. On the other hand, a thick electrode with
Nanoscale electrochemical reaction is expected to fine particles is required for having high reaction vol-
enhance the reaction by networking the nanoparticles. ume, good ionic connectivity and electrical conduct-
Furthermore, there are many possibilities to design ing grains. Therefore, controlling the structure of
366