Page 389 - Book Hosokawa Nanoparticle Technology Handbook
P. 389
6.5 ELECTROCHEMICAL PROPERTIES FUNDAMENTALS
every application and is usually estimated by com- The sensing characteristic of the oxide semiconduc-
parison of the signal intensities before and after tor can be improved by reducing the particle size to
detection. Moreover, proportional relationship nanosize. Fig. 6.5.10 shows the influence of particle
between the output signal and the quantity of the size on the sensitivity of gas detection for SnO 2
measured property is favorable in the range of the
measurement.
- 2-
6.5.2.2 Quick response O 2- O O -
O 2- O
The change of the property should be detected as 2-
quickly as possible. Also the recovery to an initial O
state after the detection should be prompt.
n-type oxide n-type oxide
semiconductor semiconductor
6.5.2.3 Selectivity
particle particle
The sensor should respond only to the property to
measure, not being affected by the other coexisting
properties.
6.5.2.4 Durability
It is necessary for the sensor to have high physical and Potential barrier
chemical stability in the actual environment. Moreover, E
it is important to endure the repetition of the detections. C
E
These characteristics are influenced by various fac- D
tors such as the material and the structure of the sen- E F
sor. Here, the effects and features of the nanostructural
control on the functions of sensors are described, sur- Surface levels
veying the characteristics of a chemical sensor with
nanostructure.
E V
(1) Characteristics of oxide semiconductor gas sensor
with nanostructure Space charge layer
An oxide semiconductor gas sensor is a practical
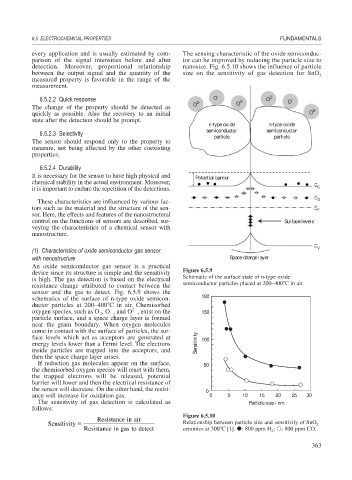
device since its structure is simple and the sensitivity Figure 6.5.9
is high. The gas detection is based on the electrical Schematic of the surface state of n-type oxide
resistance change attributed to contact between the semiconductor particles placed at 200–400 C in air.
sensor and the gas to detect. Fig. 6.5.9 shows the
schematics of the surface of n-type oxide semicon- 180
ductor particles at 200–400 C in air. Chemisorbed
2
oxygen species, such as O , O , and O , exist on the 150
2
particle surface, and a space charge layer is formed
near the grain boundary. When oxygen molecules
come in contact with the surface of particles, the sur-
face levels which act as acceptors are generated at 100
energy levels lower than a Fermi level. The electrons Sensitivity
inside particles are trapped into the acceptors, and
then the space charge layer arises.
If reduction gas molecules appear on the surface, 50
the chemisorbed oxygen species will react with them,
the trapped electrons will be released, potential
barrier will lower and then the electrical resistance of
the sensor will decrease. On the other hand, the resist- 0
ance will increase for oxidation gas. 0 5 10 15 20 25 30
The sensitivity of gas detection is calculated as Particle size / nm
follows:
Figure 6.5.10
Resistance in air
Sensitivity Relationship between particle size and sensitivity of SnO 2
Resistance in gas to detect ceramics at 300 C [1]. : 800 ppm H ; : 800 ppm CO.
2
363