Page 394 - Book Hosokawa Nanoparticle Technology Handbook
P. 394
FUNDAMENTALS CH. 6 EVALUATION METHODS FOR PROPERTIES OF NANOSTRUCTURED BODY
Recent trends on the SOFC R&D focus on: (1) the
improvement of reactivity of gas molecules and ions
at electrodes (2) the enhancement of ionic conductiv-
ity of the electrolyte. In the former case, nanoparticles
and their surrounding nanomicron scale area play an
important role in their electrochemical reactivity. As
mentioned previously, the enhancement of electro-
chemical reaction at the TPB area is most important in
the development of SOFC. Recently, the introduction
of nanotechnology with an attempt to apply nanopar-
ticles to construct nanostructure becomes the focus of
SOFC R&D.
Because of the restriction of the electrochemical
reaction, electrodes should have good ionic and elec-
trical conductivities to expedite the reaction.
Therefore, the electrode materials should exhibit a
high catalytic activity for the desired electrochemical
reactions. Nanoparticles are expected to have high
reactivity as catalysts. Over-potential in the fuel cell
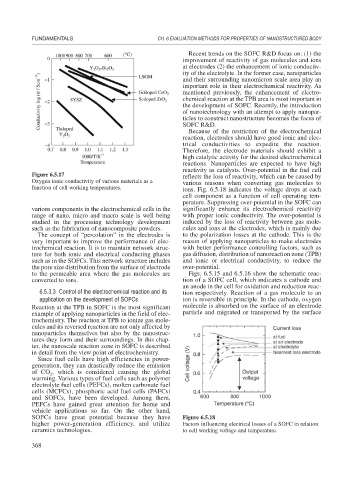
Figure 6.5.17 reflects the loss of reactivity, which can be caused by
Oxygen ionic conductivity of various materials as a various reasons when converting gas molecules to
function of cell working temperatures. ions. Fig. 6.5.18 indicates the voltage drops at each
cell component as a function of cell operating tem-
perature. Suppressing over-potential in the SOFC can
various components in the electrochemical cells in the significantly enhance its electrochemical reactivity
range of nano, micro and macro scale is well being with proper ionic conductivity. The over-potential is
studied in the processing technology development induced by the loss of reactivity between gas mole-
such as the fabrication of nanocomposite powders. cules and ions at the electrodes, which is mainly due
The concept of “percolation” in the electrodes is to the polarization losses at the cathode. This is the
very important to improve the performance of elec- reason of applying nanoparticles to make electrodes
trochemical reaction. It is to maintain network struc- with better performance controlling factors, such as
ture for both ionic and electrical conducting phases gas diffusion, distribution of nanoreaction zone (TPB)
such as in the SOFCs. This network structure includes and ionic or electrical conductivity, to reduce the
the pore size distribution from the surface of electrode over-potential.
to the permeable area where the gas molecules are Figs. 6.5.15 and 6.5.16 show the schematic reac-
converted to ions. tion of a SOFC cell, which indicates a cathode and
an anode in the cell for oxidation and reduction reac-
6.5.3.3 Control of the electrochemical reaction and its tion respectively. Reaction of a gas molecule to an
application on the development of SOFCs ion is reversible in principle. In the cathode, oxygen
Reaction at the TPB in SOFC is the most significant molecule is absorbed on the surface of an electrode
example of applying nanoparticles in the field of elec- particle and migrated or transported by the surface
trochemistry. The reaction at TPB to ionize gas mole-
cules and its reversed reaction are not only affected by
nanoparticles themselves but also by the nanostruc-
tures they form and their surroundings. In this chap-
ter, the nanoscale reaction zone in SOFC is described
in detail from the view point of electrochemistry.
Since fuel cells have high efficiencies in power-
generation, they can drastically reduce the emission
of CO , which is considered causing the global
2
warming. Various types of fuel cells such as polymer
electrolyte fuel cells (PEFCs), molten carbonate fuel
cells (MCFCs), phosphoric acid fuel cells (PAFCs)
and SOFCs, have been developed. Among them,
PEFCs have gained great attention for home and
vehicle applications so far. On the other hand,
SOFCs have great potential because they have Figure 6.5.18
higher power-generation efficiency, and utilize Factors influencing electrical losses of a SOFC in relation
ceramics technologies. to cell working voltage and temperature.
368