Page 393 - Book Hosokawa Nanoparticle Technology Handbook
P. 393
6.5 ELECTROCHEMICAL PROPERTIES FUNDAMENTALS
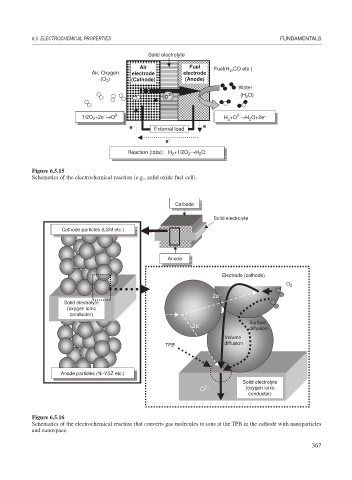
Solid electrolyte
Air Fuel Fuel(H ,CO etc.)
Air, Oxygen electrode electrode 2
(O ) (Cathode) (Anode)
2
Water
2- O)
O (H 2
−
2-
1/2O +2e →O 2- H +O →H O+2e -
2
2
2
e − External load e −
e −
Reaction (total): H +1/2O →H O
2
2
2
Figure 6.5.15
Schematics of the electrochemical reaction (e.g., solid oxide fuel cell).
Cathode
Solid electrolyte
Cathode particles (LSM etc.)
Cathode particles •LSM•
•
Anode
Electrode (cathode)
O 2
−
2e
Solid electrolyte
(oxygen ionic
conductor)
− Surface
2e
diffusion
Volume
diffusion
TPB
Anode particles (Ni-YSZ etc.)
Solid electrolyte
2− (oxygen ionic
O
conductor)
Figure 6.5.16
Schematics of the electrochemical reaction that converts gas molecules to ions at the TPB in the cathode with nanoparticles
and nanospace.
367