Page 408 - Book Hosokawa Nanoparticle Technology Handbook
P. 408
FUNDAMENTALS CH. 6 EVALUATION METHODS FOR PROPERTIES OF NANOSTRUCTURED BODY
Table 6.9.1
Gas-separation efficiencies of various polymer membranes.
10
3
10 , P cm -cm Polysulfone Acetylcellulose Polyimide Poly Silicon rubber
–2
–1
cm s cmHg (4-methylpentene-1)
H 2 13 12 9 136 550
CO 2 6 6 – 93 270
O 2 1 1 0.5 32 501
ij
H /CO 40 40 76 – –
2
H /CH 4 60 60 200 – 0.7
2
H /N 2 72 70 200 17 2
2
CO /CH 4 25 30 – – 0.3
2
O /N 2 6 5.5 4 2
2
Note: Temperature 25–30, constant pressure.
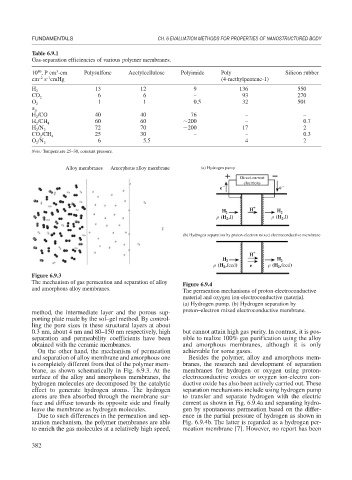
Alloy membranes Amorphous alloy membrane (a) Hydrogen pump
Direct-current
electricity
e − e −
H + H
H 2 2
(H ,I) (H ,I)
2
2
(b) Hydrogen separation by proton-electron mixed electroconductive membrane
+
H
H 2 H 2
(H ,feed) e (H ,feed)
2
2
Figure 6.9.3
The mechanism of gas permeation and separation of alloy Figure 6.9.4
and amorphous alloy membranes.
The permeation mechanisms of proton-electroconductive
material and oxygen ion-electroconductive material.
(a) Hydrogen pump. (b) Hydrogen separation by
method, the intermediate layer and the porous sup- proton–electron mixed electroconductive membrane.
porting plate made by the sol–gel method. By control-
ling the pore sizes in these structural layers at about
0.3 nm, about 4 nm and 80–150 nm respectively, high but cannot attain high gas purity. In contrast, it is pos-
separation and permeability coefficients have been sible to realize 100% gas purification using the alloy
obtained with the ceramic membranes. and amorphous membranes, although it is only
On the other hand, the mechanism of permeation achievable for some gases.
and separation of alloy membrane and amorphous one Besides the polymer, alloy and amorphous mem-
is completely different from that of the polymer mem- branes, the research and development of separation
brane, as shown schematically in Fig. 6.9.3. At the membranes for hydrogen or oxygen using proton-
surface of the alloy and amorphous membranes, the electroconductive oxides or oxygen ion-electro con-
hydrogen molecules are decomposed by the catalytic ductive oxide has also been actively carried out. These
effect to generate hydrogen atoms. The hydrogen separation mechanisms include using hydrogen pump
atoms are then absorbed through the membrane sur- to transfer and separate hydrogen with the electric
face and diffuse towards its opposite side and finally current as shown in Fig. 6.9.4a and separating hydro-
leave the membrane as hydrogen molecules. gen by spontaneous permeation based on the differ-
Due to such differences in the permeation and sep- ence in the partial pressure of hydrogen as shown in
aration mechanism, the polymer membranes are able Fig. 6.9.4b. The latter is regarded as a hydrogen per-
to enrich the gas molecules at a relatively high speed, meation membrane [7]. However, no report has been
382